Figure 3.
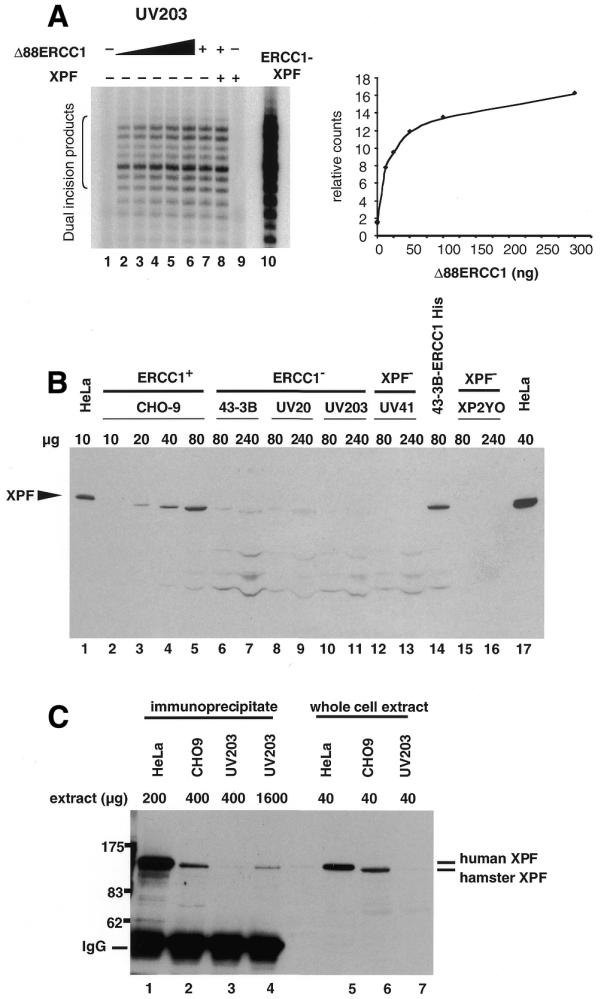
Some XPF remains in cells lacking ERCC1. (A) Extract (60 µg protein) derived from CHO UV203 ERCC1 mutant cells was used in a repair assay and complemented with increasing amounts of rΔ88ERCC1 ranging from 12.5 to 300 ng (lanes 2–6) and 38 ng in lanes 7 and 8. Reactions in lanes 8 and 9 received 260 ng of XPF. A control reaction (lane 10) was carried out with the rERCC1–XPF complex. Excised oligonucleotides are indicated by the bracket on the left. Right panel: quantification of the relative complementation levels reached with increasing amounts of rΔ88ERCC1 in lanes 1–6. (B) Extract as indicated (µg protein), from repair-proficient and mutant human and CHO cells was separated on an 8% SDS–PAGE gel. Immunodetection was carried out with XPF antibody KJ. (C) Immunoprecipitation of XPF from cell extracts. XPF antibody RA1 was used to immunoprecipitate XPF from 200 µg protein of HeLa extract (lane 1), 400 µg of normal CHO-9 extract (lane 2) and 400 (lane 3) and 1600 µg (lane 4) of ERCC1 mutant CHO UV203 extract. Immunodetection was carried out with XPF antibody KJ.
