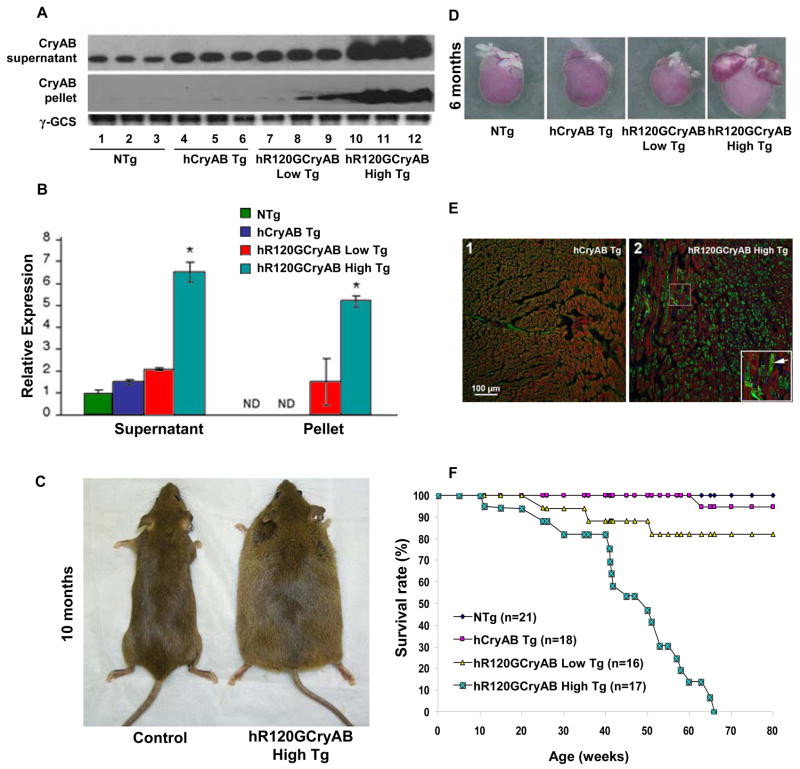
Figure 1. Cardiac-specific Overexpression of R120GCryAB Causes Protein Aggregation Cardiomyopathy in Transgenic Mice.
(A) Representative Westerns of either the soluble (supernatant) or insoluble (pellet) fractions isolated from hearts of 6 month old non-transgenic (NTg), human αB-crystallin (hCryAB Tg), hR120GCryAB Low Tg, and hR120GCryAB High Tg animals. Each lane represents an individual animal.
(B) R120GCryAB overexpression causes translocation of CryAB into the insoluble fraction in a dose-dependent manner. Fold changes are expressed in arbitrary units relative to NTg. Representative groups consist 3 or more animals (*p<0.001).
(C) Congestive heart failure exhibited by systemic edema in hR120GCryAB mice at 10 months.
(D) Human R120GCryAB overexpression causes ventricular enlargement along with biatrial thrombosis consistent with heart failure at 6 months.
(E) Indirect immunofluorescence analysis of heart sections stained with anti-CryAB detected by FITC conjugated secondary antibodies shows large protein aggregates (green) in cardiomyocytes of hR120GCryAB High Tg hearts (inset/arrow).
(F) Survival Curve. Transgenic hR120GCryAB High mice developed congestive heart failure and died between 24 and 65 weeks. Most hR120GCryAB Low Tg mice (~80%) were alive after 80 weeks. No differences in mortality were observed between hCryAB Tg and NTg littermates.