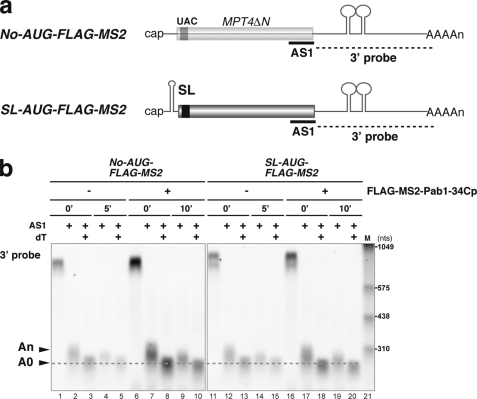
FIGURE 7.
Stabilization of non-translated mRNA by tethered Pab1–34Cp results in the accumulation of deadenylated mRNA. a, shown is a schematic drawing of the non-translated mRNAs and the antisense oligonucleotide used for the RNase H assay. The filled box indicates the open reading frame of MPT4ΔN, the black box indicates the FLAG tag, the lines represent non-translated regions, and the tract of As denotes the poly(A) tail. MS2 binding sites are indicated as stem-loop structures in the 3′-UTR. A stable stem-loop structure for inhibition of translation (SL) was introduced into the 5′-UTR of the AUG-FLAG-MS2. The probes for Northern hybridization are shown as dashed lines. The antisense oligonucleotide (AS1, 5′-CCAAAGATGGCAAGTTAGAAACGTCAATGT-3′) that was used for RNase H digestion is shown as a bold line. b, W303 cells harboring pIT2071 (No-AUG-FLAG-MS) or pIT2073 (SL-AUG-FLAG-MS2) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein. Cells were grown in SC-Gal UraLeu medium, and RNA samples were prepared as in Fig. 3a. RNA samples of cells harboring a control p415TEF1p-FLAG-MS2 plasmid were prepared before (0 min) and 5 min (5′) after a shift to glucose medium. RNA samples of cells harboring a control a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein were prepared before (0 min) and 10 min (10′) after a shift to glucose medium. RNA samples were digested with RNase H after hybridization with the AS1 oligonucleotide (AS1) or with oligo dT (dT), and Northern blot analysis was subsequently performed with a DIG-labeled 3′ probe. The RNA size marker III (11373099910, Roche Applied Science) is loaded in lane 21.