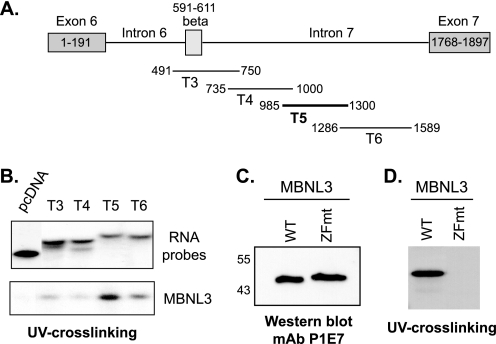
FIGURE 5.
MBNL3 directly binds to intron 7 of Mef2D pre-mRNA in vitro. A, the relative positions of uniformly radiolabeled RNA segments of Mef2D pre-mRNA transcribed in vitro for UV cross-linking experiments are shown. MBNL3 bound efficiently to the region indicated by the thick black line in vivo. B, bacterially expressed His-tagged MBNL3 was purified by nickel-agarose affinity chromatography. Purified MBNL3 was incubated with the indicated radiolabeled RNA fragments. Protein-RNA interactions were covalently cross-linked. The resulting stable complexes were separated on SDS-polyacrylamide, and protein bound to the radiolabeled probe was detected by autoradiography (lower panel). An aliquot of each RNA probe is shown (upper panel). The data presented are representative of four independent experiments. C, comparable amounts of purified WT MBNL3 and ZFmt were subjected to SDS-PAGE followed by Western blotting using the anti-MBNL3 mAb P1E7. The positions of molecular mass markers (in kDa) are shown on the left. D, RNA binding of WT and ZFmt MBNL3 proteins to the T5 fragment of Mef2D pre-mRNA was examined by UV cross-linking (n = 7) as described in B.