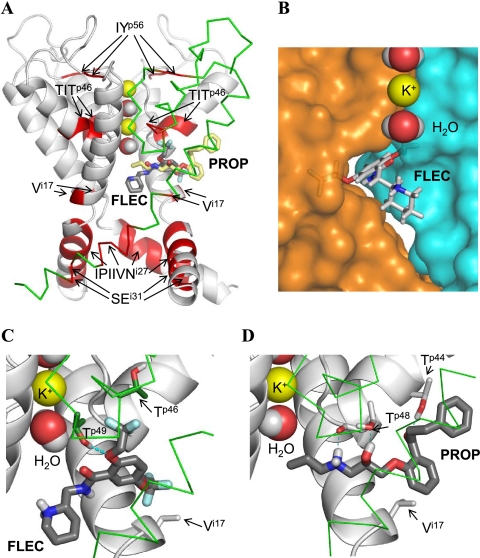
FIGURE 5.
PROP and FLEC in Kv2.1. A, overall view of the pore domain. Three subunits of the channel are shown with gray ribbons (α-helices) and gray rods. For clarity, one subunit is shown by green Cα tracing and S5 helices are not shown. K+ ions and water molecules in the selectivity filter region are space-filled. Ligands (sticks with red oxygens, blue nitrogens, dark gray carbons in FLEC, and yellow carbons in PROP) are superimposed to indicate overlapping binding sites. Channel segments whose substitutions substantially affect action of FLEC and/or PROP (Table 1 and Fig. 3) are highlighted by red. Note that segment TITp46 and residue Vi17 are close to the ligands, whereas other segments are far from the ligands (see text for further explanations). B, close-up view at FLEC in Kv2.1. The piperidine ring is in the central cavity, and the aromatic ring is in the niche between the neighboring subunits, whose surfaces are colored blue and orange. A potassium ion and two water molecules in the selectivity filter are space-filled. C and D, close-up view at FLEC and PROP, respectively, in the channel. The ligands are shown by thick sticks with dark gray carbons, red oxygens, blue nitrogens, white polar hydrogens, and cyan fluorine atoms. Channel residues, whose direct interactions with the ligands are proposed in this study, are shown by thin sticks, and their carbons atoms are colored as respective backbones. Note that FLEC interacts with the proposed residues, which are located in the neighboring subunits (gray carbons of Vi17 versus green carbons of Tp46 and Tp49), whereas PROP interacts with Tp44, Tp48, and Vi17 in the same subunit (gray carbons).