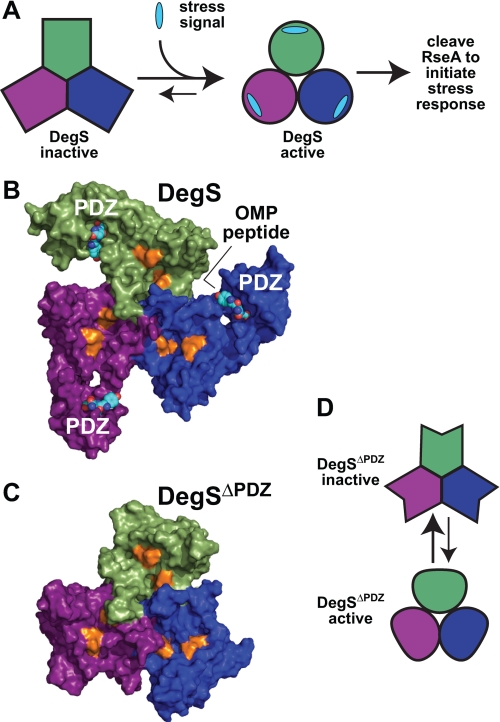
FIGURE 1.
Allosteric control of DegS activity. A, DegS equilibrates between inactive and active structures. Environmental stress produces a signal in the form of exposed OMP peptide sequences that stabilizes active DegS, resulting in cleavage of RseA and initiation of a transcriptional stress response. B, OMP peptides (shown in Corey-Pauling-Koltun representation) bind to the PDZ domains of an intact DegS trimer (surface representation; Protein Data Bank code 3GDV). The proteolytic active sites (gold color) are distant from the peptide-binding sites. C, the protease domains in DegSΔPDZ (surface representation; Protein Data Bank code 2QF3) form a stable trimer with a structure similar to the corresponding regions of peptide-activated DegS. D, model in which DegSΔPDZ also equilibrates between active and inactive conformations.