Abstract
Over 45 months, 119 angiographic examinations were performed in 95 patients prior to liver transplantation, and 53 examinations in 44 patients after transplantation. Transplantation feasibility was influenced by patency of the portal vein and inferior vena cava. Selective arterial portography, wedged hepatic venography, and transhepatic portography were used to assess the portal vein if sonography or computed tomography was inconclusive. Major indications for angiography after transplantation included early liver failure, sepsis, unexplained elevation of liver enzyme levels, and delayed bile leakage, all of which may be due to hepatic artery thrombosis. Other indications included gastrointestinal tract bleeding, hemobilia, and evaluation of portal vein patency in patients with chronic rejection who were being considered for retransplantation. Normal radiographic features of hepatic artery and portal vein reconstruction are demonstrated. Complications diagnosed using results of angiography included hepatic artery or portal vein stenoses and thromboses and pancreaticoduodenal aneurysms. Intrahepatic arterial narrowing, attenuation, slow flow, and poor filling were seen in five patients with rejection
Index terms: Liver, angiography, 952.122, 957.124, Liver, transplantation, 761.45
The first human liver transplantation was performed in 1963 (1). By 1966, six patients had received liver transplants at the University of Colorado Medical Center. Unfortunately, all of them soon died, with the longest survival being 23 days (2). The first extended survival (13 months) was achieved in 1967 (3). As survival rates have improved, transplantation has increasingly become an accepted mode of therapy for irreversible end-stage liver disease in both children and adults (4, 5). The number of transplantations performed each year has steadily increased (2, 4, 5). At the University Health Center of Pittsburgh, 265 patients received 338 orthotopic liver transplants from January 1981 to October 1984.
Possible transplantation candidates must first undergo medical evaluation (4–6), which may include angiography of the portal vein and/or inferior vena cava (IVC).
After liver transplantation, the occurrence of several complications requires angiographic evaluation. We report our experience with pre- and postoperative angiography in the evaluation of pediatric and adult liver transplantation patients.
SUBJECTS AND METHODS
Pretransplantation
During the 45 months from January 1981 to October 1984, 650 patients were evaluated at the University Health Center of Pittsburgh to determine the feasibility of transplantation. Ninety-five patients (14.6%) required a total of 119 angiographic studies, involving the portal vein in 84, the IVC in two, and both vessels in nine. Of the 11 patients requiring evaluation of the IVC, nine were children in whom congenital absence of the cava was suspected. To evaluate the portal vein, selective superior mesenteric, splenic, and/or hepatic arteriography was performed. If selective arterial portography was inconclusive, wedged hepatic venography was performed; three patients underwent direct transhepatic portography. In most cases, wedged hepatic venography was performed on another day so as not to exceed contrast limitations.
Posttransplantation
During the same period, 265 patients (119 children and 146 adults) received a total of 338 orthotopic liver transplants; 53 received two transplants each, and ten received three transplants each. There were 125 males and 140 females, with an age range of 4 months to 56 years.
Following the procedure, 44 patients (23 children and 21 adults) underwent 53 angiographic studies. This included three patients who underwent transplantation in Denver prior to the beginning of the Pittsburgh program. Seven patients were studied twice, and one patient was studied three times. Angiography was performed from 2 days to 11 years and 8 months after transplantation. Approximately 70% of the studies were performed within the first 2 months following transplantation. Indications for angiography are given in Table 1.
Table 1.
Indications for Angiography Following Liver Transplantation
| Children | Adults | |
|---|---|---|
| Possible hepatic artery thrombosis | 17 | 13 |
| Possible portal vein thrombosis | 3 | 1 |
| GI tract bleeding | 6 | 3 |
| Hemobilia | 2 | 3 |
| Chronic rejection; need to evaluate the portal vein for retransplantation | 2 | 3 |
| Total | 30 | 23 |
Liver homograft revascularization consisted of anastomoses of the hepatic artery, portal vein, and IVC. The hepatic artery was reconstructed in one of several ways. In most cases the end of the donor’s celiac axis or common hepatic artery was anastomosed to the end of the recipient’s common or proper hepatic artery (Fig. 1). Several patients had an iliac artery homograft obtained from the donor (Fig. 2). In several children, the donor’s thoracic or abdominal aorta (together with the celiac axis) was anastomosed to the side of the recipient’s abdominal aorta (Fig. 3). In a few patients whose native common hepatic artery arose from the superior mesenteric artery, the donor’s celiac axis was anastomosed to the end of the common hepatic artery.
Figure 1.

Homograft arterial revascularizatlon by direct end-to-end anastomosis. Selective celiac arteriogram shows a patent anastomosis (curved arrow) between the donor’s celiac axis and the recipient’s common hepatic artery, the most common method of reconstruction. The ligated donor splenic (straight arrow) and gastroduodenal arteries (arrowhead) are also shown.
Figure 2.

Patent iliac artery homograft (small arrows) and hepatic artery. The proximal anastomosis is between the common iliac end of the graft and the recipient’s lower abdominal aorta (curved arrow), while the distal anastomosis is from the external iliac end of the graft to the donor’s common hepatic artery (straight arrow). The ligated donor internal iliac artery is also shown (arrowhead).
Figure 3.
Homograft arterial revascularization using the donor’s aorta. The end of the thoracic or (as in this case) abdominal aorta (straight arrow), together with the celiac axis, is anastomosed to the side of the recipient’s lower abdominal aorta (curved arrow).
Anomalies of the donor’s hepatic artery required a different type of anastomosis, depending on the anomaly (7). The most common variant was origin of an accessory right hepatic artery (or the entire right hepatic artery) from the superior mesenteric artery, coupled with origin of the left hepatic artery from the celiac axis. Vascular reconstruction in adults is illustrated in Figure 4.
Figure 4.
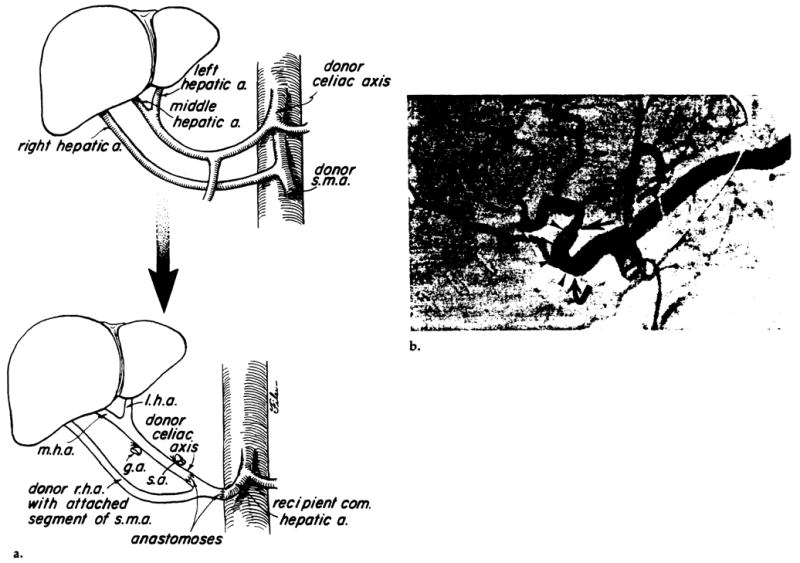
Arterial revascularization of a liver homograft with a dual blood supply. (a) The donor’s liver is supplied by both the celiac axis (via the left and middle hepatic arteries) and the superior mesenteric artery (s.m.a) (via the right hepatic artery). At the time of hepatectomy a small segment of the s.m.a. is removed together with the right hepatic artery. The proximal end of the s.m.a. is anastomosed to the end of the recipient’s common hepatic artery, and the distal end is anastomosed to the end of the donor’s celiac axis. r.h.a. = right hepatic artery, m.h.a. = middle hepatic artery, l.h.a. = left hepatic artery, g.a. = gastroduodenal artery, s.a. = splenic artery. (b) Selective celiac arteriogram demonstrated patent proximal (curved arrow) and distal anastomoses (straight arrow) to the donor’s superior mesenteric artery segment (arrowheads).
The portal vein was usually revascularized by end-to-end anastomosis of the extrahepatic portal veins of the donor and recipient (Fig. 5). In a few patients with an abnormally small or occluded portal vein, a venous homograft from the donor’s IVC, iliac, or pulmonary vein was interposed between the donor’s extrahepatic portal vein and the confluence of the recipient’s superior mesenteric and splenic veins (8) (Fig. 6).
Figure 5.

Portal vein reconstruction. Venous phase of the selective superior mesenteric arteriogram reveals a widely patent end-to-end anastomosis (curved arrow) between the donor and recipient extrahepatic portal veins. Note the minimal “waist” at the anastomotic line.
Figure 6.
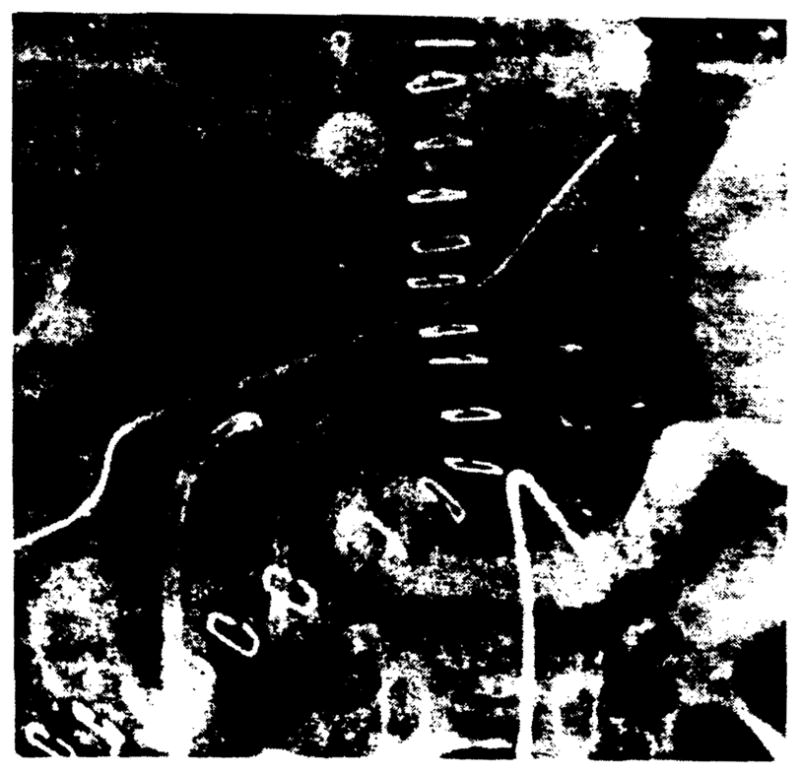
Portal vein reconstruction using a venous homograft. The graft (small arrows) is anastomosed between the donor’s extrahepatic portal vein (curved arrow) and the confluence of the recipient’S superior mesenteric and splenic veins (straight arrow) as demonstrated on the venous phase of the selective splenic arteriogram. A small segment of the donor’s IVC was used for the graft.
Reconstruction of the IVC consisted of anastomoses of the infra- and suprahepatic vena caval segments of both donor and recipient.
RESULTS
Pretransplantation
The portal vein was patent in 78 of 93 patients (84%). In 12 patients, selective superior mesenteric, splenic, and hepatic angiography were inconclusive regarding portal vein patency; however, wedged hepatic venography confirmed patency in eight (Fig. 7). Three patients underwent direct transhepatic portography, which confirmed patency in one.
Figure 7.

Patent portal vein (arrows) demonstrated by wedged hepatic venography. The coronary vein is also shown (arrowhead).
Four of nine children evaluated with inferior vena cavography were found to have congenital absence of the IVC with azygos continuation to the heart. Thrombosis of the IVC was found in two adults.
Posttransplantation
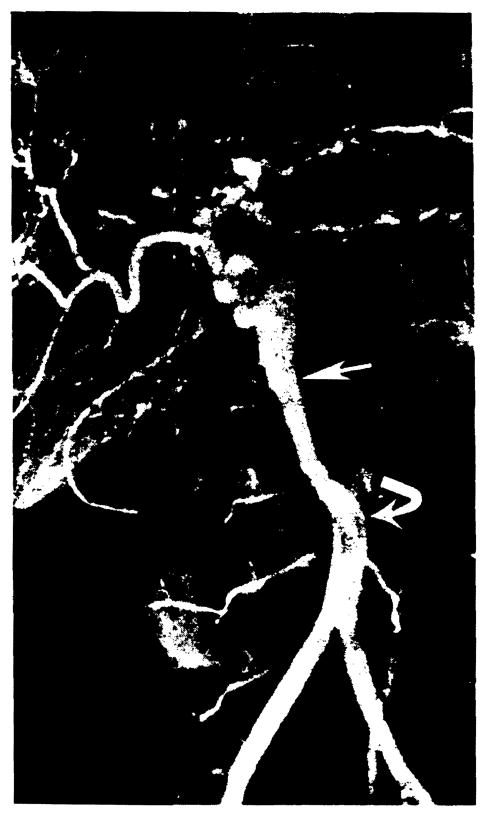
Twenty-six of 53 angiograms were normal or showed no significant abnormality (Table 2). Hepatic artery thrombosis occurred in seven children and one adult 10 days to 5 weeks after transplantation. Four of these patients had the hepatic artery reconstructed by end-to-end anastomosis of the donor and recipient hepatic arteries, and four had vascular homografts (two aortic and two iliac). In one child whose hepatic artery became thrombosed 2 weeks after transplantation (Fig. 8a), a follow-up angiogram 6 weeks later showed arterial collaterals to the transplanted liver (Fig. 8b).
Table 2.
Angiographic Findings Following Liver transplantation
| Children | Adults | |
|---|---|---|
| Normal | 14 | 12 |
| Hepatic artery thrombosis | 7 | 1 |
| Hepatic artery stenosis | 0 | 4 |
| Portal vein thrombosis* | 4 | 0 |
| Portal vein stenosis | 3 | 1 |
| Pancreticobuobenal aneurysms | 0 | 1 |
| Intrahepatic arterial narrowing, alow flow, attenuation, poor filling | 1 | 4 |
| Arterial collaterals to the liver after hepatic artery thrombosis | 1 | 0 |
Reconstruction of the Intrahepatic portal vessels via periportal venous colla6teral (cavernomatous transformation) occurred in two patients (Fig. 10).
Figure 8.
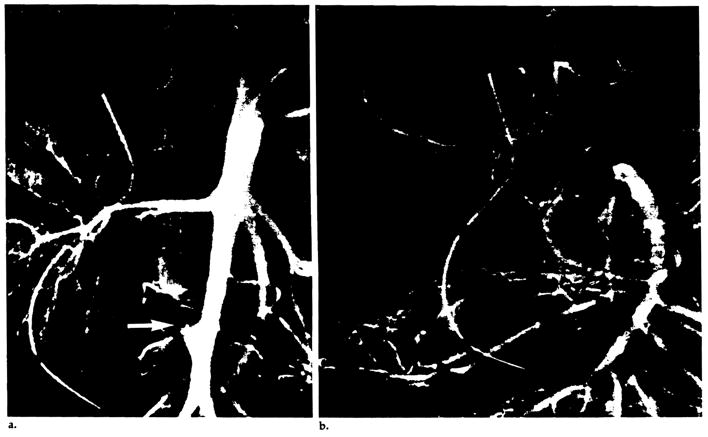
Collateral development following hepatic artery thrombosis. (a) Thrombosis of the iliac artery homograft (arrow) is seen on an abdominal aortogram obtained 2 weeks after transplantation. (b) Six weeks later, a selective superior mesenteric arteriogram demonstrates intrahepatic arterial reconstitution (arrows) via collaterals (arrowheads).
Hepatic artery stenosis was observed in three transplants; two occurred at the arterial anastomosis. In one patient, angiography demonstrated a stenosis, which proved at exploratory laparotomy to be a kink in the artery. The artery was straightened, but the stenosis was not believed to be hemodynamically Significant. In one patient who had a follow-up angiogram 5 months later, the anastomotic stenosis had essentially resolved. In another patient with an anastomotic stenosis (Fig. 9), percutaneous balloon angioplasty reduced the pressure gradient from 40 to 20 mm Hg. However, the liver was rejected, and retransplantation was required.
Figure 9.

Anastomotic stenosis (arrow) demonstrated by selective hepatic arteriography 10 days after transplantation. A pressure gradient of 40 mm Hg was recorded across the stenosis.
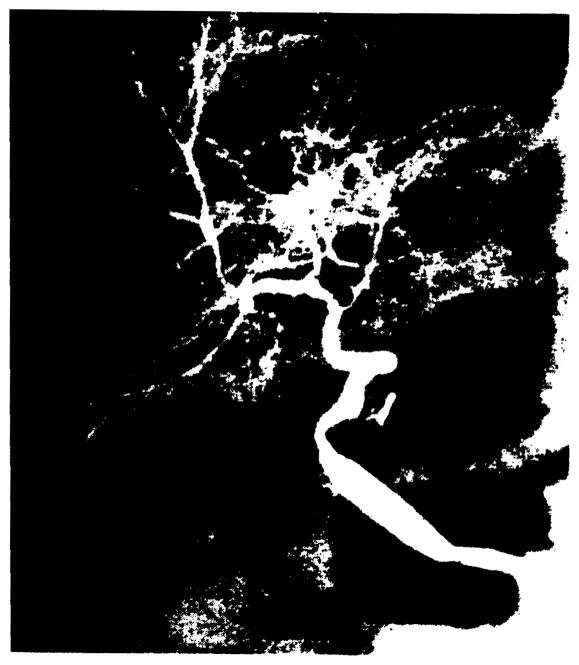
Three patients had portal vein occlusion and presented with upper gastrointestinal (GI) tract bleeding. Occlusion developed after 8 days in one patient, and retransplantation was required. Angiography demonstrated massive gastroesophageal varices and no filling of intrahepatic portal vessels. In two patients, occlusion developed 1 year after transplantation. Angiographically, both patients demonstrated complete occlusion of the extrahepatic portal vein associated with massive gastroesophageal varices, but the intrahepatic portal veins were reconstituted via numerous periportal collaterals (Fig. 10).
Figure 10.

Collateral development following portal vein thrombosis. Venous phase of the selective superior mesenteric arteriogram 1 year after transplantation reveals occlusion of the extrahepatic portal vein and reconstitution of the intrahepatic portal vessels (straight arrows) by massive periportal collaterals (arrowheads). Marked gastric varices are also present (curved arrow).
Portal vein stenosis occurred in three children and one adult at 2, 4, 20, and 32 weeks after transplantation, respectively (Fig. 11). All presented with upper GI tract bleeding.
Figure 11.
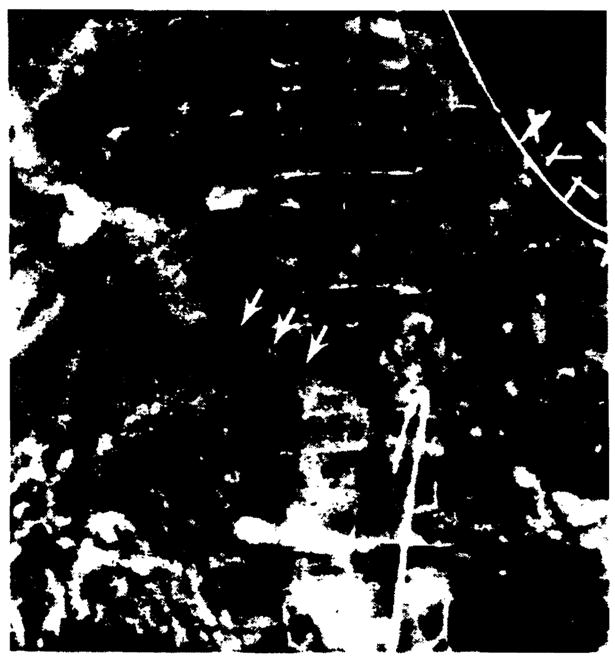
Marked portal vein narrowing (arrows) demonstrated on venous phase of a selective superior mesenteric arteriogram. Gastric and esophageal varices are also present.
In one patient who was evaluated for hemobilia 10 weeks after transplantation, multiple aneurysms were observed in pancreaticoduodenal collateral vessels (Fig. 12). At laparotomy, the aneurysms were found to be mycotic with erosion into the common bile duct.
Figure 12.
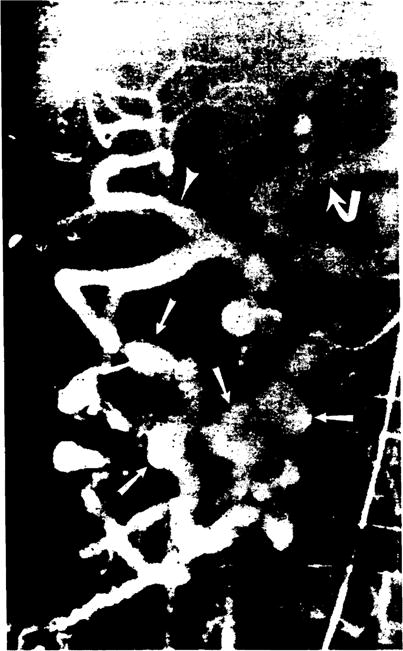
Mycotic aneurysms (straight arrows) in pancreaticoduodenal arterial collaterals demonstrated on a selective superior mesenteric arteriogram. The hepatic (arrowhead) and splenic (curved arrow) arteries are also filled.
One patient with pathologically proved chronic rejection was evaluated for retransplantation after 8 months. Diffuse and focal areas of narrowing were present within the hepatic arterial tree (Fig. 13). Four other patients with clinical evidence of rejection demonstrated slowing of flow, poor peripheral filling, and attenuation in the size and number of arterial branches within the liver.
Figure 13.
Chronic rejection. Diffuse and segmental intrahepatic arterial narrowing is demonstrated on a selective hepatic arteriogram taken 8 months after transplantation.
DISCUSSION
The increased success rate of liver transplantation over the past 20 years is due to many factors. Among the most important are improved surgical technique, changes in patient selection, use of the immunosuppressive drug cyclosporine, and better postoperative care (9,10).
Each patient with an acceptable indication for liver transplantation undergoes a preoperative medical evaluation, which generally requires a 5–7-day hospitalization. The portal vein is examined by sonography and/or dynamic computed tomography (CT) to determine patency. It is considered normal on sonography if the walls are smooth and there are no internal echoes. If the portal vein cannot be seen because of overlying gas or altered venous anatomy as the result of previous surgery, if it is smaller than 4 mm, or if there are internal portal vein echoes suggesting thrombus, angiography should be performed. Angiography is also indicated if there is evidence of portal vein occlusion on dynamic CT. Doppler ultrasound, which will be available in the near future, should be invaluable in examining portal vein blood flow prior to transplantation.
In most patients, selective superior mesenteric or splenic arteriography with venous-phase filming will demonstrate the portal vein. However, many of these patients have severe portal hypertension with hepatofugal portal venous flow. In some, the portal vein may not be visualized after superior mesenteric or splenic arteriography. Occasionally, selective hepatic arteriography will show the portal vein in patients with complete reversal of flow in the portal vein. If selective arterial studies are inconclusive, wedged hepatic venography should be performed (Fig. 7). Only three of our patients required direct transhepatic portography.
Until recently, patency of the portal vein was a prerequisite for liver transplantation (6) because of the type of end-to-end anastomosis performed for portal venous revascularization (Fig. 5). With improved surgical techniques, however, transplantation is still feasible even if the recipient’s portal vein is thrombosed or abnormally small in diameter (8). If it is less than 3 mm or appears occluded on angiography, the confluence between the superior mesenteric and splenic veins is evaluated. If the junction between these two veins is patent and of reasonable size, the donor’s portal vein can be anastomosed to it using an interposed vascular homograft (Fig. 6). At the time of donor hepatectomy, segments of the aorta, IVC, and iliac arteries and veins are removed; then if a vascular graft is needed during transplantation, these homograft vessels are readily available for hepatic artery, portal vein, or IVC reconstruction (11).
The other major indication for pretransplantation angiography is evaluation of children with suspected congenital absence of the IVC. The major indication for transplantation in the pediatric age group is biliary atresia (4), which is associated with a higher frequency of congenital absence of the intrahepatic IVC, as well as other vascular and visceral anomalies (12). Although such absence is not an absolute contraindication to transplantation, when combined with other hepatic vascular anomalies it represents a high-risk group of patients who should be identified prior to surgery.
Vascular anomalies in these children include aberrant origin of the hepatic artery and preduodenal portal vein, while visceral anomalies include situs inversus, polysplenia, and GI tract malrotation (12). Prior to transplantation, most patients with biliary atresia have undergone a portoenterostomy (Kasai procedure) for biliary drainage (13), which frequently reveals visceral or vascular anomalies. In addition, an upper GI tract contrast study will have been performed to exclude malrotation. To identify this high risk group of patients, therefore, all patients with biliary atresia and GI tract malrotation or a visceral or vascular anomaly undergo inferior vena cavography to evaluate for possible congenital absence of the IVC.
At our center, the procedure of choice for hepatic artery reconstruction in patients with a normal native hepatic artery of sufficient size is end-to-end anastomosis of the donor’s celiac axis to the recipient’s common hepatic artery (14) (Fig. 1), which assumes a single arterial supply to the donor liver. Other types of anastomoses depend on the vascular anatomy of the donor and recipient (7). In children with very small hepatic arteries in whom a direct hepatic artery anastomosis may be difficult, the donor’s thoracic or abdominal aorta, in continuity with the celiac axis, is anastomosed to the recipient’s abdominal aorta (Fig. 3). Usually the abdominal aorta is preferred, but it is frequently unavailable because the kidneys and part of the abdominal aorta are harvested for renal transplantation at the time the donor’s liver is removed. In children given a liver with a dual blood supply (e.g., left hepatic artery from the celiac axis and right hepatic artery from the superior mesenteric artery), the thoracic or abdominal aorta (in continuity with both vessels) has been used for arterial revascularization. In adults, however, an anastomosis as shown in Figure 4 is usually employed. An iliac artery homograft may be used when the recipient’s hepatic artery is small (or absent), occluded, has poor flow, or if subintimal dissection develops during vascular mobilization (Fig. 2). An iliac homograft may also be placed between the donor and recipient hepatic arteries when they are too short to permit a direct end-to-end anastomosis.
The main reason for posttransplant angiography is suspected hepatic artery thrombosis, which is a major indication for early retransplantation (15). Clinical findings include early hepatic failure, sepsis, unexplained elevation of liver enzyme levels, and delayed bile leakage. Such leakage may be secondary to necrosis of the distal donor bile duct, and revision of the biliary anastomosis is necessary. However, major bile leakage has been associated with complete necrosis of the donor bile duct secondary to hepatic artery thrombosis. In these patients retransplantation, rather than biliary reconstruction, is always necessary (16).
Postoperatively, CT or ultrasound may be helpful in patients with sepsis or fever if there is a clinical suspicion of an abdominal abscess. Patients with focal alterations in the liver parenchyma (infarct or abscess) with these noninvasive imaging tests have an 85% incidence of hepatic artery thrombosis (17).
While hepatic artery occlusion may be tolerated by non-liver-transplantation patients, following transplantation it can result in hepatic gangrene or even death because of the lack of potential for arterial collateralization to the liver. With proximal hepatic artery occlusion in a nontransplant liver, collaterals develop rapidly, preventing ischemia and infarction (18–20). Because of this, the survival of the liver probably cannot be solely attributable to a patent portal venous system (21). Since all potential collateral pathways are severed at the time of transplantation, occlusion of the hepatic artery after transplantation completely dearterializes the liver, possibly resulting in severe or even fatal hepatic injury.
Once thrombosis is diagnosed, retransplantation is performed as soon as a donor liver is available. Most of our patients died if a new liver was not immediately available. However, three patients did survive, and in one of them, a follow-up angiogram 6 weeks later showed arterial collaterals supplying the liver (Fig. 8). This patient is currently alive after 1 year. The two additional patients surviving following hepatic artery thrombosis did not have repeat angiography, and we presume that they also have collaterals.
In one patient with hepatic artery thrombosis and liver failure, a donor liver was not available, and low-dose streptokinase was infused into the occluded hepatic artery. After several hours, GI tract bleeding occurred, and streptokinase was discontinued. However, the patient continued to bleed and died. At autopsy, there was diffuse hemorrhage into the GI tract but no focal bleeding points. Streptokinase is not routinely used after hepatic artery thrombosis because liver infarction is usually present once the diagnosis has been established. Infection often supervenes in these immunosuppressed patients and may lead to septic hepatic gangrene. In such cases, emergency retransplantation is the only hope for survival (15). In addition, these patients have recently undergone surgery and are therefore at a higher risk of bleeding as a result of streptokinase therapy.
Other indications for angiography following transplantation include GI tract bleeding, hemobilia, and evaluation of portal vein patency in patients with chronic rejection who are being considered for retransplantation. In patients with GI tract bleeding in whom endoscopy demonstrates varices, angiography of the portal venous system is indicated. Several of our patients demonstrated either stenosis or occlusion of the portal vein. One patient with stenosis underwent successful revision of the portal vein anastomosis; another patient who had occlusion required retransplantation. Two patients demonstrated typical cavernomatous transformation of the portal vein (Fig. 10). The mechanism by which these venous collaterals develop is unknown, since all potential collaterals are severed during the operation. One of these patients who had persistent upper GI tract bleeding that failed to respond to endoscopic esophageal sclerotherapy was treated with a distal splenorenal shunt for control of portal hypertension. The other patient is being treated by means of endoscopic sclerotherapy.
In the three patients with hemobilia, angiography was performed to evaluate for possible pseudoaneurysm at the arterial anastomosis. In none of these patients was a false aneurysm found. In one case, however, the donor’s hepatic artery was anastomosed to the recipient’s proper hepatic artery, and the liver was supplied by enlarged pancreaticoduodenal collaterals from the superior mesenteric artery, which had presumably developed in response to stenosis or occlusion of the native celiac axis. In addition, these collateral vessels demonstrated multiple aneurysms (Fig. 12). At exploratory laparotomy, these aneurysms were found to be mycotic in origin and to have eroded into the com mom bile duct. These aneurysms were resected, and an iliac artery homograft was placed. Seven days later, the patient died after massive GI tract and intraabdominal bleeding developed due to complete septic disruption of the iliac artery homograft.
The diagnosis of rejection is generally made by exclusion. The patient may present with elevated liver enzyme and serum bilirubin levels; other causes include biliary obstruction, ischemic damage, viral infection, and hepatic artery thrombosis (22). Biliary obstruction can be excluded by cholangiography, and vascular thrombosis can be ruled out by angiography. In our series, several patients with clinical rejection demonstrated slowing of flow, poor peripheral filling, and attenuation in the size and number of the intrahepatic arteries. Analogous findings have been observed in the biliary tree after transplantation (16). In one patient with biopsyproved chronic rejection, severe diffuse and focal arterial narrowing was demonstrated (Fig. 13). Pathologically, these arterial lesions are due to deposition of subintimal foam cells, intimal sclerosis, and myointimal hyperplasia (23). The end result is severe narrowing or occlusion of the vessels (23). The radiographic-pathologic correlation of rejection will be the subject of a future report.
Acknowledgments
We thank Donna Scahill for manuscript preparation and Ron Filer for artwork.
Footnotes
Presented at the 70th Scientific Assembly and Annual Meeting of the Radiological Society of North America, Washington, D.C., November 25–30, 1984
References
- 1.Starzl TE, Marchioro TL, von Kaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–414. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gartner JC, Jr, Zitelli BJ, Malatack JJ, Shaw BW, Iwatsuki S, Starzl TE. Orthotopic liver transplantation in children: two-year experience with 47 patients. Pediatrics. 1984;74:140–145. [PMC free article] [PubMed] [Google Scholar]
- 5.Van Thiel DH, Schade RR, Gavaler JS, Shaw BW, Jr, Iwatsuki S, Starzl TE. Medical aspects of liver transplantation. Hepatology. 1984;4 (suppl 1):79S–83S. doi: 10.1002/hep.1840040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Thiel DH, Schade RR, Starzl TE, et al. Liver transplantation in adults. Hepatology. 1982;2:637–640. doi: 10.1002/hep.1840020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw BW, Jr, Iwatsuki S, Starzl TE. Alternative methods of arterialization of the hepatic graft. Surg Gynecol Obstet. 1984;159:490–493. [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw BW, Jr, Iwatsuki S, Starzl TE. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet. in press. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Koep LJ, Halgrimson CG, et al. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 10.Calne RY, Williams R, Lindop M, et al. Improved survival after orthotopic liver grafting. Br Med J [Clin Res] 1981;283:115–118. doi: 10.1136/bmj.283.6284.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Halgrimson CG, Koep LJ, Weil R, III, Taylor PD. Vascular homografts from cadaveric organ donors. Surg Gynecol Obstet. 1979;149:737. [PMC free article] [PubMed] [Google Scholar]
- 12.Lilly JR, Starzl TE. Liver transplantation in children with biliary atresia and vascular anomalies. J Pediatr Surg. 1974;9:707–713. doi: 10.1016/0022-3468(74)90109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasai M, Suzuki H, Ohashi E, Ohi R, Chiba T, Okamoto A. Technique and results of operative management of biliary atresia. World J Surg. 1978;2:571–579. doi: 10.1007/BF01556048. [DOI] [PubMed] [Google Scholar]
- 14.Starzl TE. Experience in hepatic transplantation. Chapt 8. Philadelphia: Saunders; 1969. The recipient operation in man; pp. 112–143. [Google Scholar]
- 15.Shaw BW, Jr, Gordon RD, Iwatsuki S, Starzl TE. Hepatic retransplantation. Transplant Proc. 1985;17:264–271. [PMC free article] [PubMed] [Google Scholar]
- 16.Zajko AB, Campbell WL, Bron KM, et al. Cholangiography and interventional biliary radiology in adult liver transplantation. AJR. 1985;144:127–133. doi: 10.2214/ajr.144.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segel MC, Zajko AB, Bowen A, et al. Hepatic artery thrombosis after liver transplantation: radiologic evaluation. AJR. doi: 10.2214/ajr.146.1.137. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mays ET, Wheeler CS. Demonstration of collateral arterial flow after interruption of hepatic arteries in man. N Engl J Med. 1974;290:993–996. doi: 10.1056/NEJM197405022901804. [DOI] [PubMed] [Google Scholar]
- 19.Bengmark S, Rosengren K. Angiographic study of the collateral circulation to the liver after ligation of the hepatic artery in man. Am J Surg. 1970;119:620–624. doi: 10.1016/0002-9610(70)90228-x. [DOI] [PubMed] [Google Scholar]
- 20.Redman HC, Reuter SR. Arterial collaterals in the liver hilus. Radiology. 1970;94:575–579. doi: 10.1148/94.3.575. [DOI] [PubMed] [Google Scholar]
- 21.Doppman JL, Girton M, Kahn ER. Proximal versus peripheral hepatic artery embolization: experimental study in monkeys. Radiology. 1978;128:577–588. doi: 10.1148/128.3.577. [DOI] [PubMed] [Google Scholar]
- 22.Pichlmavr R, Brölsch C, Neuhaus P, et al. Report on 68 human orthotopic liver transplantations with special reference to rejection phenomena. Transplant Proc. 1983;15:1279–1283. [Google Scholar]
- 23.Demetris AJ, Lasky S, Van Thiel DH, Starzl TE, Dekker A. Pathology of hepatic transplantation. A review of 62 adult allograft recipients immunosuppressed with a cyclosporine/steroid regimen. Am J Pathol. 1985;118:151–161. [PMC free article] [PubMed] [Google Scholar]