Abstract
On the basis of observations in patients with long-term (28–30 years) renal allograft survival, all of whom had evidence of systemic microchimerism, we began a program of combined simultaneous kidney/bone marrow transplantation. Between 12/14/92, and 10/31/94, 36 kidney transplant recipients received 3–5 × 108 unmodified bone marrow cells/kg; 6 patients also received pancreatic islets, and 7 patients also received a pancreas. The mean recipient age was 39.0 ± 10.8 years, and the mean donor age was 31.8 ± 16.1 years; the mean cold ischemia time was 23.0 ± 9.1 hr. Twenty control patients received kidneys alone, mainly because of refusal by the donor family to consent to vertebral body recovery; 3 of these patients also received a pancreas. The mean recipient age was 47.9 ± 11.7 years, and the mean donor age was 41.5 ± 17.9 years; the mean cold ischemia time was 28.6 ± 6.2 hr. All patients received tacrolimus-based therapy, without radiation, cytoreduction, or induction antilymphocyte preparations. Blood was drawn prior to and at regular intervals after transplantation for detection of chimerism and for immunologic studies. With a mean follow-up of 11.1 ± 5.8 months, all 36 study patients are alive, and 33 (92%) have functioning allografts with a mean serum creatinine of 1.9 ± 1.2 mg/dl and a BUN of 26 ± 9 mg/dl. Graft vs. host disease was not seen in any patient. The incidence of rejection was 72%; 11% of the patients required OKT3 or ATG for steroid-resistant rejection. The incidence of CMV was 14%, and that of delayed graft function was 17%. A total of 18 (90%) control patients are alive, and 17 (85%) have functioning allografts, with a mean serum creatinine of 2.1 ± 1.3 mg/dl, and a BUN of 30 ± 13 mg/dl. The incidence of rejection was 60%, and 10% required OKT3 or ATG. CMV was seen in 15%, and delayed graft function in 20% (P=NS). In the study patients, chimerism was detected in the peripheral blood of 30 of 31 (97%) evaluable patients by either PCR or flow cytometry. In the control patients, chimerism was seen in 9 of 14 (64%) evaluable patients (P<.02). Decreasing donor-specific responsiveness was seen in 6/29 (21%) evaluable study, and 4/14 (29%) evaluable control patients (P=NS). We conclude that combined kidney/bone marrow transplantation is associated with acceptable patient and graft survival, augmentation of chimerism, and no change in the early events after transplantation.
The discovery that systemic microchimerism is present in renal transplant recipients with stable, long-term (28–30 years) graft survival (1) has led to the theory that chimerism may be required for successful long-term engraftment (2–4). These observations resulted in trials of donor bone marrow infusion at the time of renal (or other solid organ) transplantation, with the goal of augmenting levels of chimerism and improving long-term patient and graft survival (5–7). An early report of the first ten kidney recipients showed reasonable short-term patient and graft survival with routine augmentation of chimerism and no evidence of graft vs. host disease (GVHD) (8). The incidence of rejection, delayed graft function, and cytomegalovirus was not different from that observed in control patients who did not receive bone marrow. The present report discusses the outcome in the first 36 patients undergoing combined kidney/bone marrow transplantation.
MATERIALS AND METHODS
Between December 17, 1992, and October 31, 1994, 36 patients underwent combined, simultaneous kidney/bone marrow transplantation. Six patients also received islets, and 7 also received a pancreas, in each case from the same donor. The mean recipient age was 39.0 ± 10.8 years (range 18.7–63.4). Two (6%) patients were undergoing retransplantation, and one (3%) had a panel-reactive antibody level (PRA) greater than 40%. One (3%) patient received a living related donor kidney and bone marrow from a 1 haplotype–matched brother; the remainder received kidneys and bone marrow from cadaveric donors. whose mean age was 31.8 ± 16.1 years (range 7.0–60). The mean number of HLA matches and mismatches was 2.3 ± 1.3 and 3,4 ± 1.5, respectively, and the mean cold ischemia time was 23.0 ± 9.1 hr (range 7.5–44.2).
Twenty control patients underwent kidney transplantation alone: the most common reason for the lack of availability of donor bone marrow was family refusal to give consent for vertebral body recovery. Three patients also received a pancreas. All patients were undergoing their first cadaveric transplantation, and one (5%) had a PRA over 40%. These patients and their donors were somewhat older: the mean recipient age was 47.9 ± 11.7 years (range 29.7–66.3; P<.01, compared with the kidney/bone marrow recipients). The mean donor age was 41.5 ± 17.9 years (range 5.0–67.0; P<.05, compared with the kidney/bone marrow group). The mean numbers of HLA matches and mismatches were 2.6 ± 1.3 and 3.4 ± 1.4 (P=ns compared with the kidney/bone marrow group), and the mean cold ischemia time was 28.6 ± 6.2 hrs (range 17.8–37.9, P<.02 compared with the kidney/bone marrow recipients). Some of these differences were related to the large number of diabetes undergoing islet or pancreas transplantation in the bone marrow group. When kidney-only recipients were considered, the bone marrow and control patients were not statistically different in terms of donor or recipient age, or cold ischemia time.
Immunosuppression was with tacrolimus and steroids, as previously described (9). Induction antilymphocyte therapy, cytoreduction, or radiation therapy was not given to any patient.
Bone marrow isolation
Bone marrow, in all but one case, was recovered from the donor vertebral bodies, the method for which has been described elsewhere (5). Briefly, the vertebral bodies were broken up into small cancellous bone chips and placed in a processing medium (8) for 30–60 min, during which time the bone marrow cells were passively released. The cell suspension was then filtered and centrifuged at 300 ×g for 11 min, and the pellet was placed in a suspension medium (8). The cell number and viability were determined by trypan blue dye exclusion. The cells were diluted with the suspension medium to a concentration of 2×107 cells/ml, and then refrigerated at 4°C until the recipient was ready. At that point, the cell suspension was centrifuged and resuspended in 200 ml of the suspension medium, and given intravenously over a period of 20–30 min.
In the living related case, donor bone marrow was aspirated from the iliac crest after completion of the donor nephrectomy and processed in a standard manner (10). In all cases, 3–5 × 108 unmodified donor bone marrow cells/kg were given intravenously at the conclusion of the kidney transplant procedure.
Chimerism and in vitro immune testing studies
Just prior to and at regular intervals following transplantation, blood was drawn for detection of chimerism by fluorescent activated cell sorter analysis (FACS), and polymerase chain reaction (PCR). Serial in vitro immune monitoring was also performed using mixed lymphocyte reactions and assays to determine reactivity to recall antigens and mitogens.
FACS
In all immunostaining procedures, mouse-antihuman monoclonal antibodies (mAb) directed against the polymorphic epitopes of HLA class I were used to distinguish donor from recipient HLA alleles. These primary mAb were labeled with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated goat-antimouse secondary antibodies. The specificity and the optimal dilution of each were determined by staining donor spleen cells and the recipient’s pretransplant peripheral blood mononuclear cells (PBMC). To delineate leukocyte lineages, PE or FITC-conjugated mouse-antihuman mAbs directed against different cell surface receptors were used. Isotype-matched irrelevant mAbs were used as negative controls. FACS was feasible in 25 of the 31 study patients and 12 of the 18 control cases. Frequency of cells <0.5% was considered below the reliable detection threshold.
PCR
A search for chimerism was feasible in 24 of the 31 study cases and 11 of 18 control patients using oligonucleotides of either the sex determining region of the Y-chromosome (SRY) or the appropriate mismatched HLA alleles as primers. The DNA was amplified and resolved by electrophoresis on an agarose gel. After Southern blotting, the membrane was hybridized with a specific radiolabeled probe, exposed to a film, and developed.
In-vitro immune testing
The in-vitro immune status of the recipients before and after transplantation was determined by the response of recipient’s PBMC to ConA and PHA mitogens and recall antigen, and mixed lymphocyte reaction (MLR). In these unidirectional human MLR cultures, gamma-irradiated donor splenocytes, and third-party PBMC were used as stimulators (105 cells/well). The cells were cultured at 37°C for 6 days in 5% CO2 in air; for the final 20 hr [3H] thymidine [1μCi] was added to each well and its degree of incorporation was determined by liquid scintillation counting.
Pancreatic islets were infused into a branch of the portal vein after completion of the renal transplant procedure. Islet preparation was according to previously described techniques (11, 12). Similarly, the pancreas transplant was performed either immediately before or after the renal transplant procedure, using standard techniques (13).
Statistical analysis
The standard two-sample t test was used to test differences in means, while differences in rates and proportions were tested using Pearson’s chi-square test of association. A P-value less than 0.05 was considered statistically significant.
RESULTS
The mean follow-up was 11.1 ± 5.8 months (Table 1). All kidney/bone marrow recipients were alive, and 33 (92%) had functioning allografts. Two patients lost their allografts 16 months after transplantation, one to noncompliance and the other to rejection. One additional patient lost his allograft 12.5 months after transplantation to a combination of polyoma (B-K) virus infection and rejection. The mean serum creatinine and BUN were 1.9 ± 1.2 mg/dl and 26 ± 9 mg/dl. Rejection, which was documented histologically in all cases, was seen in 26 (72%) patients—however, only 4 (11%) patients required antilymphocyte therapy.
Table 1.
Outcome after kidney transplantation with and without bone marrow augmentation
| Kidney/bone marrow (n=36) | Control (n = 20) | |
|---|---|---|
| Follow-up | 11.1 ±5.8 Months | |
| Patient survival | 100% | 90% |
| Graft survival | 92% | 85% |
| Serum creatinine (mg/dl) | 1.9±1.2 | 2.1±1.3 |
| BUN (mg/dl) | 26± 9 | 30±13 |
| Rejection | 26 (72%) | 12 (60%) |
| OKT3 or ATG | 4 (11%) | 2 (10%) |
| Tacrolimus dose (mg/dl) | 10.0±6.0 | 8.0±5.0 |
| Tacrolimus level (ng/ml) | 10.4±4.4 | 8.8±4.3 |
| Off steroids | 13 (39%) | 8 (47%) |
| Cytomegalovirus | 5 (14%) | 3 (15%) |
| Delayed graft function | 6 (17%) | 4 (20%) |
| Graft vs. host disease | 0 (0%) | 0 (0%) |
| Chimerism | 30/31 (97%)a | 9/14 (64%) |
| Decreasing donor-specific responsiveness | 6/29 (21%) | 4/14 (29%) |
P<.02, all other comparisons, P=NS.
The mean tacrolimus dosage was 10.0 ± 6.0 mg/d, and the level (whole blood IMX) was 10.4 ± 4.4 ng/ml; 13 (39%) patients were off steroids, and the mean prednisone dosage for the patients still on steroids was 6.1 ± 3.3 mg/d.
In the control group, 18 (90%) patients were alive; one patient died of sepsis 2 months after transplantation, having lost his kidney for technical reasons 1 month earlier. Another patient died of suspected hyperkalemia 5 months after transplantation, having at that time lost her allograft to rejection. An additional patient lost her allograft two weeks after transplantation to vascular rejection; thus 17 (85%) of the control patients have functioning allografts. The mean serum creatinine and BUN were 2.1 ± 1.3 mg/dl and 30 ± 13 mg/dl. Rejection was seen in 12 (60%) patients; 2 (10%) patients required antilymphocyte therapy.
The mean tacrolimus dosage was 8.0 ± 5.0 mg/d, and the level was 8.8 ± 4.3 ng/ml: 8 (47%) of the patients were off steroids, and the mean prednisone dosage for those patients still on steroids was 6.1 ± 2.5 mg/d.
Cytomegalovirus was diagnosed in 5 (14%) kidney/bone marrow and 3 (15%) control patients, all of whom received and responded to intravenous gancyclovir. Delayed graft function was observed in 6 (17%) kidney/bone marrow and 4 (20%) control patients (P=ns compared with kidney/bone marrow patients for all the above survival, function, dosage, and complication data).
Graft-versus-host disease was not observed in any patient (14).
Thirty-one of the 33 kidney/bone marrow patients with functioning grafts were evaluable for chimerism by flow cytometry and/or PCR. The others could not be evaluated because of a perfect DR match or a lack of donor/recipient sex disparity. As expected, chimerism was not detectable in the pretransplant blood specimen of any patient. At most recent follow-up after transplantation, 30 (97%) kidney/bone marrow patients had evidence of persistent peripheral blood chimerism by one or more modality (Fig. 1 and 2). Of 14 control patients who were evaluable for chimerism, 9 (64%) were chimeric (P<.02). In general, the level of chimerism appeared to be higher in the bone marrow–augmented group than in the control patients.
Figure 1.
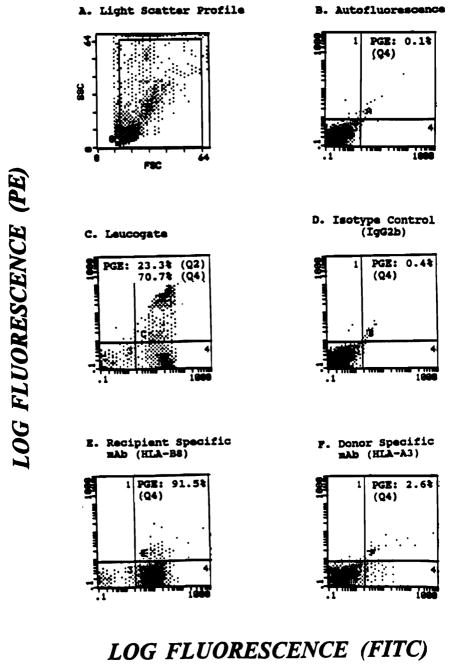
Flow cytometric analysis of a kidney and bone marrow recipient 260 d posttransplantation. Cells stained with donor (HLA-A3) or recipient (HLA-B8) specific mAb were acquired using wide gates on a light scatter profile (A). (E) Recipient cells (91.5%) (F) Donor cells (2.6%). Unstained (B) and cells stained with isotype-matched irrelevant mAb (D) were used as controls. Axes: (C) CD45—X, CD14—Y; (D) IgG 2b—X; (E) HLA-B8—X; (F) HLA-A3—X.
Figure 2.

Detection of donor DNA by PCR in the peripheral blood of two female recipients of kidney and bone marrow from male donors for up to 484 (II) and 261 (III) days posttransplantation. (I) Titration curve to determine the sensitivity of the detection of the SRY-region of the Y-chromosome; log dilutions from 100 to 0.001 ng in lanes A to F respectively. (II) Evidence for the presence of donor DNA on POD 252 (A), 266 (B), 428 (C), and 484 (D). (III) Donor DNA was also detected in another BM-augmented kidney + islet recipient on D15 (A), 163 (B), and 261 (C) posttransplantation.
Serial mixed lymphocyte reaction (MLR) evaluation was performed to look for decreasing donor-specific responsiveness. This was seen in 6 of 29 (21%) evaluable kidney/bone marrow recipients and 4 of 14 (29%) evaluable control patients (P=NS [Table 1]). There was evidence of a correlation between an increasing responsiveness and rejection in two patients who went on to lose their allografts to rejection. One of the cases was the patient who lost his kidney to noncompliance, who had evidence of donor-specific hyporesponsiveness prior to discontinuing his immunosuppression.
DISCUSSION
Bone marrow augmentation is in some sense a conceptual descendant of pretransplant donor-specific blood transfusions, although many of the details are obviously different (15). Common to both ideas is the goal of improving long-term graft survival by immunomodulation. Our data suggest that bone marrow augmentation is associated with acceptable patient (100%) and graft (92%) survival, no graft-versus-host disease, and a similar incidence of routinely observed post-transplant complications. when compared with the control group. Chimerism was observed more frequently (97%) and at a higher level than in the control group (64%). Thus bone marrow augmentation was not harmful and was associated with an increase in chimerism. It should be noted that this was not a randomized trial and that the control group was older, both in terms of recipient and donor age, and had a longer cold ischemia time. It took nearly two years to accrue 36 patients, and over one-third had the allocation advantage of receiving islets or a whole pancreas. The average annual number of kidney transplantations during this period was 200—thus a very small percentage of patients was able to be entered into this trial. To perform a randomized trial in this circumstance would not have allowed for any sort of meaningful case accrual. The disparity in age and cold ischemia time is a reflection of the disproportionate number of diabetics receiving islets or a whole pancreas; these patients tend to be younger than the average renal transplant patient, and the ischemia time tends to be shorter as well. When we compared the 23 kidney-only bone marrow patients with the 17 kidney-only control patients, the mean donor and recipient age and cold ischemia time were not statistically different.
The long-term questions in the kidney/bone marrow patients await more follow-up. Improvement in long-term graft survival, persistence of chimerism, and an increase in donor-specific hyporesponsiveness are all issues that will need to be studied over the next several years to establish the utility of bone marrow augmentation. Questions about the optimal way to perform bone marrow augmentation are still being addressed. Our regimen of a single perioperative dose of bone marrow and no induction antilymphocyte therapy, cytoreduction, or radiation represents the most straightforward approach. Other approaches have included delayed administration with induction antilymphocyte therapy (16–20), pretransplant radiation therapy and modification of the bone marrow (21–23). and administration of more than one dose of donor bone marrow (Tzakis A, Miller J, Ricordi C., personal communication). The long-term assessment of these different approaches will take years, and it thus may not be straight-forward to establish which regimen will be the most useful. What our study has demonstrated is that short-term patient and graft survival are acceptable, that there is augmentation of the incidence and level of chimerism, and that the procedure is safe, with no evidence of graft-versus-host disease. These findings confirm our earlier reports in a larger number of patients. Accrual of cases in our program is continuing. Routine vertebral body recovery in other centers may allow for more widespread application of bone marrow augmentation and may facilitate sharing of organs and bone marrow between different centers.
Acknowledgments
We would like to thank Jareen Flohr, R.N., B.S.N., Loraine Kaminski, R.N., Regina Fenton, R.N., B.S.N., C.C.T.C., Deborah Good, R.N., B.S.N., C.C.T.C., Holly Woods, R.N., C.C.T.C., Sue Bauder, R.N., Janice Zagari, R.N., B.S.N., Jennifer Ovesney, R.N., B.S.N., Mark Paynter, R.N., B.S.N., and Sharon Orlofske, R.N., for their help with patient care; Ilene Felser, R.N., for help with patient follow-up; Janet Schmelzer, Jo Harnaha, and Merrit Lutz for their help with data entry and organization; Mark Braun and Troy Seskey for assistance in the isolation of bone marrow and in vitro analysis of chimerism; Sue Lombardozzi, M.D., Mary Pavlick, and Rick Banas for assistance with in vitro immune monitoring; Alison Logar for assistance in performing now cytometry; Kate Carr for her help with slide preparation; and Karen Toler for her help with typing the manuscript and with table and slide preparation.
Footnotes
Presented at the 14th Annual Meeting of the American Society of Transplant Physicians, May 15–17, 1995, Chicago, IL.
References
- 1.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism and donor-specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1272. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Cell chimerism permitted by immunosuppressive drugs is the basis of organ transplant acceptance and tolerance. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontes P, Abdul R, Demetris AJ, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao AS, Fontes P, Zeevi A, et al. Combined bone marrow and whole organ transplantation from the same donor. Transplant Proc. 1994;26:3377. [PMC free article] [PubMed] [Google Scholar]
- 7.Rao AS, Fontes P, Zeevi A, et al. Augmentation of chimerism in whole organ recipients by simultaneous infusion of donor bone marrow cells. Transplant Proc. 1995;27:210. [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro R, Rao AS, Fontes P, et al. Combined kidney/bone marrow transplantation-evidence for augmentation of chimerism. Transplantation. 1995;59:306. doi: 10.1097/00007890-199501000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro R, Jordan ML, Scantlebury VP, et al. A prospective, randomized trial of FK506 in renal transplantation—a comparison between double and triple drug therapy. Clin Transplant. 1994;8:508. [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas ED, Storb R. Technique for human marrow grafting. Blood. 1970;36:507. [PubMed] [Google Scholar]
- 11.Ricordi C, Lacy PE, Finkle EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 12.Ricordi C, Tzakis A, Carroll P, et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplantation. 1992;53:407. doi: 10.1097/00007890-199202010-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyden G, Groth C. Pancreas transplantation. In: Starzl TE, Shapiro R, Simmons R, editors. Atlas of organ transplantation. New York: Gower; 1992. p. 8.4. [Google Scholar]
- 14.Deeg HJ, Storb R. Graft versus host disease: pathophysiological and clinical aspects. Annu Rev Med. 1984;35:11. doi: 10.1146/annurev.me.35.020184.000303. [DOI] [PubMed] [Google Scholar]
- 15.Salvatierra O, Jr, Melzer J, Potter D, et al. A seven-year experience with donor-specific blood transfusions: results and considerations for maximum efficacy. Transplantation. 1985;40:654. doi: 10.1097/00007890-198512000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Caridis DT, Liegeois A, Barrett I, Monaco AP. Enhanced survival of canine renal allografts of ALS-treated dogs given bone marrow. Transplant Proc. 1973;5:671. [PubMed] [Google Scholar]
- 17.Thomas FT, Carver M, Foil MB, et al. Long-term incompatible kidney survival in outbred higher primates without chronic immunosuppression. Ann Surg. 1983;198:370. doi: 10.1097/00000658-198309000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monaco AP, Clark AW, Brown RW. Active enhancement of a human cadaver renal allograft with ALS and donor bone marrow: case report of an initial attempt. Surgery. 1976;79:384. [PubMed] [Google Scholar]
- 19.Barber WH, Mankin JA, Laskow DA, et al. Long-term results of a controlled prospective study with transfusion of donor specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel DO, Naftilan J, Hulvey K, et al. Peripheral blood chimerism in renal allograft recipients transfused with donor bone marrow. Transplantation. 1994;57:852. doi: 10.1097/00007890-199403270-00014. [DOI] [PubMed] [Google Scholar]
- 21.Main JM, Prehn RT. Successful skin homografts after the administration of high dosage × radiation and homologous bone marrow. J Natl Cancer Inst. 1955;15:1023. [PubMed] [Google Scholar]
- 22.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ildstad S, Sachs DHG. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]