Abstract
We identified adpA as an araC-like regulatory gene needed for colonial morphogenesis in Streptomyces coelicolor and showed that its activity depended on a unique TTA triplet corresponding to the leucyl-tRNA gene (bldA). These findings partially explained the dependence of aerial mycelium formation on a rare tRNA that is postulated to have developmental control functions.
Streptomyces species are multicellular bacteria that undergo a program of colonial development that may depend on a translational regulatory system. bldA, encoding the principal tRNA for translation of the TTA (leucine) codon, is dispensable for vegetative growth but is conditionally required at a later stage for the synthesis of aerial mycelium (in all species tested) and for the biosynthesis of some antibiotics (11, 12); for a recent analysis and additional citations, see reference 18. There is evidence that accumulation of the active form of bldA tRNA to maximal levels occurs later, at the onset of antibiotic biosynthesis and morphological differentiation (13, 19). The biosynthesis of the antibiotics actinorhodin (blue) and undecylprodigiosin (red) is bldA dependent (5, 20) and is activated by the pathway-specific activator genes actII-orfIVand redZ, both containing TTA codons. For actII-orfIV, it has been shown that replacement of the TTA codon by an alternative leucine codon relieves its dependence on bldA and thus allows actinorhodin biosynthesis in bldA mutants (5); comparable results have been obtained after mutagenesis of TTA codons in several antibiotic resistance genes (12). However, at least in some contexts and under certain nutritional conditions, TTA triplets are mistranslated and can provide active gene products in bldA mutants (6, 12, 18). The target gene (or genes) and corresponding TTA codon underlying the aerial mycelium defect in bldA mutants have not been identified.
In Streptomyces griseus, both aerial hypha formation and biosynthesis of streptomycin rely on adpA, a gene containing a TTA codon and encoding an AraC-like transcriptional regulator (17). This triplet is found at the corresponding position in all known adpA orthologs (S. coelicolor [1], S. griseus [17], and S. avermitilis [7]), suggesting that adpA may be a bldA-dependent regulator of aerial hypha formation conserved within Streptomyces species. This model was explored in the experiments described here (similar results have been obtained by E. Takano, M. Tao, F. Long, Maureen J. Bibb, L. Wang, W. Li, M. J. Buttner, Mervyn J. Bibb, Z. X. Deng, and K. F. Chater, personal communication).
adpA, a regulator of aerial hypha formation and antibiotic biosynthesis.
A 1.2-kb fragment encoding the S. coelicolor AdpA protein (Swiss-Prot/TrEMBL entry name Q9L062) was amplified by PCR with primers adpA-N-NdeI (GGGCTTAGCCATATGAGCCACGACTCCA; the engineered NdeI site containing the presumed ATG start codon is underlined) and adpA-C-BamHI (GGATCCGAGCCGTCTGCTCACCTCACG; the engineered BamHI site is underlined) and cloned into pGEM-T-Easy (Promega), and its sequence was verified. To mutagenize the gene, an apramycin resistance gene cassette on an SmaI fragment (3) was introduced into the unique MscI site in its central region. A 3.0-kb fragment carrying disrupted adpA was excised by EcoRI digestion, cloned into the same site in pSET151 (2), a vector carrying the thiostrepton resistance gene, and used to disrupt adpA in wild-type strain J1501 (by transformation) (10). An apramycin-resistant, thiostrepton-sensitive colony containing disrupted adpA, as confirmed by PCR and Southern blot analyses, was isolated. Inactivation of adpA dramatically slowed colonial development on several media (R2YE and MS [10]). Compared to parent strain J1501, the adpA mutant (Fig. 1A and 2) was impaired in its ability to form aerial mycelium (bald), and individual colonies produced more red pigment on R2YE medium. Possible effects on blue pigment synthesis could not be determined, since our isolate of parent strain J1501 produced very little actinorhodin under these conditions.
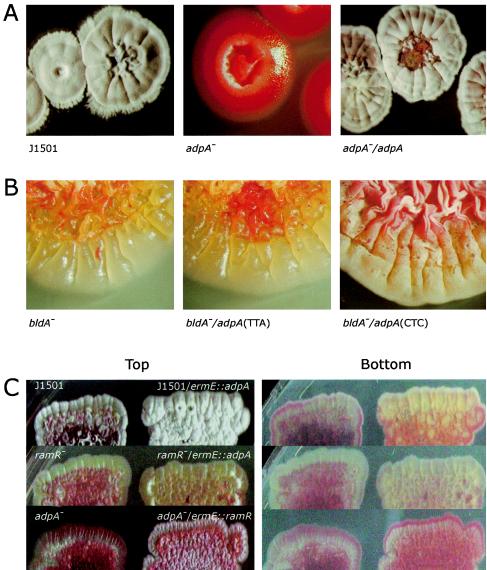
FIG. 1.
bldA-dependent translation of adpA (bldH) is required for colonial morphogenesis. (A) The wild type (J1501) (left panel), the adpA mutant (middle panel), or the adpA mutant transformed with plasmid pIJ904::adpA(TTA) carrying the intact wild-type gene (right panel) was grown on R2YE medium at 30°C for 5 days. Colonies produced no diffusible blue pigment; the darker appearance of the agar reflects photographic conditions that were adjusted for the optimal exposure of colonies having different levels of brightness. (B) The bldA39 mutant strain (left panel) or the bldA39 mutant strain containing a wild-type (middle panel) or a mutant (right panel) allele cloned in pIJ904 was grown on R2YE medium at 30°C for 2 weeks. (C) Strains that were wild type (J1501) or defective for aerial mycelium formation (ramR or adpA mutants) (left panels) were transformed with plasmids containing wild-type ramR (ermE::ramR) or adpA (ermE::adpA) genes cloned into vector pHM11A to allow expression from the strong constitutive ermE promoter (right panels). Cultures were grown on R2YE medium at 30°C for 1 week and photographed from the top or bottom.
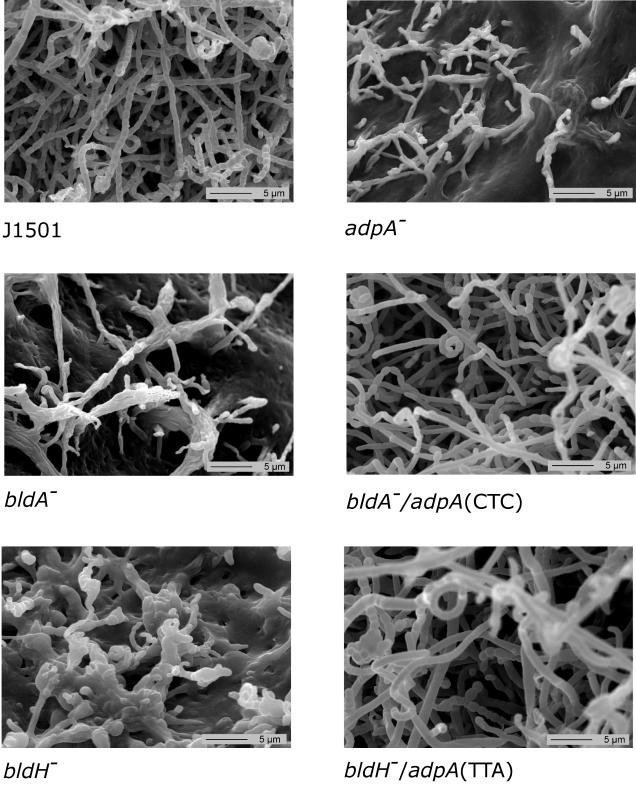
FIG. 2.
bldA-dependent translation of adpA (bldH) is required for aerial mycelium formation. Scanning electron microscopic views (14) of colonial surface cultures grown on R2YE medium for 10 days at 30°C are shown. adpA and bldH mutant strains were transformed with vector pIJ904 carrying the indicated adpA alleles [adpA(CTC), adpA allele with the CTC mutation; adpA(TTA), adpA allele with wild-type TTA].
For complementation studies, a 1.6-kb fragment carrying the promoter and coding region of adpA was amplified by PCR with primers adpA-pro1-HindIII/BamHI (GGATCCAAGCTTGGGAAAAAGCACCGGTCGACTGAC) and adpA-C-BamHI (see above) and cloned into the BamHI site of pIJ904 [pIJ904::adpA(TTA)]. Alternatively, to express adpA from the strong constitutive ermE promoter, the adpA coding region was PCR amplified with primers adpA-N-NdeI and adpA-C-BamHI and cloned into pHM11A between the NdeI and BamHI sites [pHM11A::adpA(TTA)]. In the adpA mutant, both of these plasmids suppressed the defect in aerial mycelium formation [the results obtained with pIJ904::adpA(TTA) are shown in Fig. 1A]. adpA overexpression by pHM11A::adpA(TTA) in the wild-type strain (J1501) induced rapid aerial hypha formation but permanently suppressed pigmentation (Fig. 1C). These results, along with the enhanced red pigmentation of adpA mutant colonies, showed that adpA was needed to activate morphological differentiation and could suppress synthesis of the red-pigmented antibiotic (undecylprodigiosin) in S. coelicolor. However, the inverse relationship between aerial mycelium formation and pigment biosynthesis related to adpA expression may be indirect, representing competing processes that may or may not have biological relevance.
adpA and bldH109 are mutant alleles of the same gene.
While an adpA ortholog is present in the S. coelicolor genome, a corresponding adpA mutant allele has not been found among known developmental genes. Several lines of evidence suggested that bldH, one of the few unidentified mutant genes, may be such an allele. Intercellular complementation analyses placed bldH and bldA alleles in the same group, suggesting that they are required for the same developmental functions (21). Consistent with this suggestion, bldA tRNA may be needed to translate the S. coelicolor adpA gene, since it contains a rare TTA codon (1). Initial evidence that bldH does indeed correspond to adpA was provided by experiments showing that the cloned adpA gene [pIJ904::adpA(TTA)] was able to complement the bldH109 mutant (Fig. 2). Finally, when the adpA locus from the bldH109 mutant was amplified by three independent PCRs, cloned separately, and sequenced, each was found to contain an additional G residue inserted at position +221 with respect to the annotated coding frame. We conclude that bldH109 is a frameshift mutation in the adpA gene.
Transcription and translation of adpA.
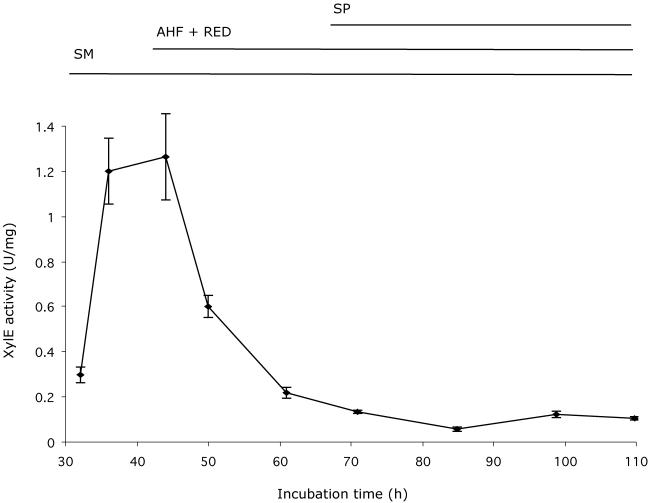
To monitor the promoter activity of the adpA upstream sequence, it was fused to the reporter gene, xylE, in vector pXE4 (pXE4::padpA). A 0.5-kb fragment covering the adpA promoter region was amplified by PCR with primers adpA-pro1-HindIII/BamHI and adpA-pro2/BamHI (GGATCCCAGCACCGCGACGATCTCCTTG) and cloned into the HindIII and BamHI sites of pXE4 (10), generating a fusion with xylE (8). XylE enzyme activity increased markedly just before and during aerial hypha formation and then gradually decreased (Fig. 3). Thus, the adpA promoter was apparently under developmental control, and the timing of its expression was coordinated with aerial hypha formation.
FIG. 3.
adpA promoter activity is developmentally regulated. Pregerminated spores (106) of wild-type strain J1501/pXE4 and strain J1501/pXE4::padpA were plated on thiostrepton-containing R2YE medium, covered with cellophane membranes, and incubated at 30°C. Extracts were prepared as described previously (15), and catechol dioxygenase activity was calculated as the rate of change in the optical density at 375 nm per minute, expressed as specific activity (units per milligram of protein; standard deviation bars are based on assays of three biological replicate samples). The XylE activity of the control strain carrying only vector pXE4 was insignificant (<0.005 U/mg) (data not shown). SM, substrate mycelium; AHF, aerial hypha formation; RED, undecylprodigiosin production; SP, sporulation.
To test whether the translation of adpA mRNA was dependent on bldA, perhaps related to the accumulation of an active form of bldA tRNA (13, 19), the unique TTA codon in adpA was changed to CTC. The mutant allele was generated by a two-stage PCR mutagenesis approach (15) with primers adpA-pro1-HindIII/BamHI and adpA-C-BamHI together with primers adpA/CTC1 (ACAGGTCTCTCCCGGAGGAGATCG; the mutated codon is underlined) and adpA/CTC2 (TCTCCTCCGGGAGAGACCTGTCGA; the mutated codon is underlined). The PCR product was cloned into pGEM-T-Easy, and its sequence was verified. The insert was removed by BamHI digestion and cloned into the BamHI site of pIJ904 [pIJ904::adpA(CTC)]. In the bldA mutant background, the adpA allele with the CTC mutation (Fig. 1B) was able to induce aerial hypha formation (Fig. 1B). In contrast, the cloned wild-type TTA-containing allele had no apparent effect on the bldA mutant (Fig. 1B). Scanning electron microscopic inspection (Fig. 2) of the colony surface showed that pIJ904::adpA(CTC) allowed the bldA strain to form some aerial hyphae and spores having a wild-type appearance, while the wild-type construct [pIJ904::adpA(TTA)] had no effect (data not shown). The fact that aerial hypha formation in the pIJ904::adpA(CTC)-complemented bldA strain was significantly delayed and reduced compared to that in the wild-type strain could have been due to defective transcriptional control of the cloned gene. However, it may indicate the requirement for additional bldA targets that facilitate aerial hypha formation, including TTA-containing genes outside the adpA locus. Finally, it is also possible that, in the bldA mutant background, the mutant CTC codon facilitated translation of the cloned adpA copy but translational arrest at the native TTA-containing chromosomal copy had a polar effect on the expression of other developmental genes located downstream.
adpA activity requires ramR.
ramR is a response regulator gene within a cluster of genes needed for aerial hypha formation in both S. coelicolor (15, 16) and S. griseus (amfR) (17). In S. coelicolor, its overexpression accelerates aerial mycelium formation in wild-type strains and restores aerial hypha formation in most defective mutants, including bldA and bldH mutants. ramR promoter activity is dependent on several developmental genes, including the bldA and bldH genes (9). Thus, ramR apparently serves as one of the final steps of the regulatory cascade leading to aerial hypha formation (15).
Like the overexpression of ramR, the overexpression of adpA in wild-type S. coelicolor induced rapid aerial mycelium formation (Fig. 1C). The fact that this effect was not significant in the ramR mutant (Fig. 1C) suggested that adpA activity was dependent on ramR. In the complementary experiment, the overexpression of ramR in either adpA (Fig. 1C) or bldH (15) mutants was able to accelerate aerial hypha formation. These results provide additional evidence that ramR functions downstream of adpA or bldH.
Concluding remarks.
We have presented evidence suggesting that adpA transcription is activated during aerial hypha formation and that its TTA triplet is required for aerial mycelium formation. However, we reemphasize that these and other studies of bldA do not prove that the activity of a specific tRNA reflects a bona fida regulatory system (18) or that it is necessarily peculiar to developmental systems in Streptomyces. Interestingly, a recent comparison of the levels of charged tRNAs that carry the same amino acid (isoacceptors) relative to the nonrandom distributions of their corresponding codons in Escherichia coli genes suggested that the selective use of alternative isoacceptors can regulate certain functions (4). The model predicts that under amino acid starvation conditions, charged tRNA isoacceptors corresponding to preferred codons will be depleted first and that those corresponding to rare codons will remain relatively abundant. Selective codon usage in genes encoding enzymes involved in amino acid biosynthesis or their corresponding regulatory elements (attenuators) suggests that it may provide translational regulatory functions during starvation. By analogy, developmental genes containing the rare TTA codon in Streptomyces may be preferentially translated during starvation responses associated with antibiotic biosynthesis and colonial morphogenesis.
Acknowledgments
This work was supported by Swiss National Science Foundation grants 31-59156.99 and 3100AO-100293/1.
We thank D. Fink, S. P. Salvatore, and K. Chater for sharing information prior to publication, Marcus Dürrenberger and Marcel Dueggelin for electron micrographs, and Urs Jenal for critical reading of the manuscript.
REFERENCES
- 1.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 2.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 3.Blondelet Rouault, M. H., J. Weiser, A. Lebrihi, P. Branny, and J. L. Pernodet. 1997. Antibiotic resistance gene cassettes derived from the omega interposon for use in E. coli and Streptomyces. Gene 190:315-317. [DOI] [PubMed] [Google Scholar]
- 4.Elf, J., D. Nilsson, T. Tenson, and M. Ehrenberg. 2003. Selective charging of tRNA isoacceptors explains patterns of codon usage. Science 300:1718-1722. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Moreno, M. A., J. L. Caballero, D. A. Hopwood, and F. Malpartida. 1991. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell 66:769-780. [DOI] [PubMed] [Google Scholar]
- 6.Gramajo, H. C., E. Takano, and M. J. Bibb. 1993. Stationary-phase production of the antibiotic actinorhodin in Streptomyces coelicolor A3(2) is transcriptionally regulated. Mol. Microbiol. 7:837-845. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda, H., J. Ishikawa, A. Hanamoto, M. Shinose, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, and S. Omura. 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21:526-531. [DOI] [PubMed] [Google Scholar]
- 8.Ingram, C., M. Brawner, P. Youngman, and J. Westpheling. 1989. xylE functions as an efficient reporter gene in Streptomyces spp.: use for the study of galP1, a catabolite-controlled promoter. J. Bacteriol. 171:6617-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keijser, B. J., G. P. Van Wezel, G. W. Canters, and E. Vijgenboom. 2002. Developmental regulation of the Streptomyces lividans ram genes: involvement of RamR in regulation of the ramCSAB operon. J. Bacteriol. 184:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 11.Lawlor, E. J., H. A. Baylis, and K. F. Chater. 1987. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2). Genes Dev. 1:1305-1310. [DOI] [PubMed] [Google Scholar]
- 12.Leskiw, B. K., E. J. Lawlor, J. M. Fernandez-Abalos, and K. F. Chater. 1991. TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative, Streptomyces mutants. Proc. Natl. Acad. Sci. USA 88:2461-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leskiw, B. K., R. Mah, E. J. Lawlor, and K. F. Chater. 1993. Accumulation of bldA-specified tRNA is temporally regulated in Streptomyces coelicolor A3(2). J. Bacteriol. 175:1995-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller, T., R. Guggenheim, M. Düggelin, and C. Scheidegger. 1991. Freeze-fracturing for conventional and field emission low-temperature scanning electron microscopy: the scanning cryo unit SCU 020. J. Microsc. 161:73-83. [Google Scholar]
- 15.Nguyen, K. T., J. M. Willey, L. D. Nguyen, L. T. Nguyen, P. H. Viollier, and C. J. Thompson. 2002. A central regulator of morphological differentiation in the multicellular bacterium Streptomyces coelicolor. Mol. Microbiol. 46:1223-1238. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor, T. J., P. Kanellis, and J. R. Nodwell. 2002. The ramC gene is required for morphogenesis in Streptomyces coelicolor and expressed in a cell type-specific manner under the direct control of RamR. Mol. Microbiol. 45:45-57. [DOI] [PubMed] [Google Scholar]
- 17.Ohnishi, Y., S. Kameyama, H. Onaka, and S. Horinouchi. 1999. The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: identification of a target gene of the A-factor receptor. Mol. Microbiol. 34:102-111. [DOI] [PubMed] [Google Scholar]
- 18.Trepanier, N. K., S. E. Jensen, D. C. Alexander, and B. K. Leskiw. 2002. The positive activator of cephamycin C and clavulanic acid production in Streptomyces clavuligerus is mistranslated in a bldA mutant. Microbiology 148:643-656. [DOI] [PubMed] [Google Scholar]
- 19.Trepanier, N. K., G. D. Nguyen, P. J. Leedell, and B. K. Leskiw. 1997. Use of polymerase chain reaction to identify a leucyl tRNA in Streptomyces coelicolor. Gene 193:59-63. [DOI] [PubMed] [Google Scholar]
- 20.White, J., and M. Bibb. 1997. bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J. Bacteriol. 179:627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willey, J., J. Schwedock, and R. Losick. 1993. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 7:895-903. [DOI] [PubMed] [Google Scholar]