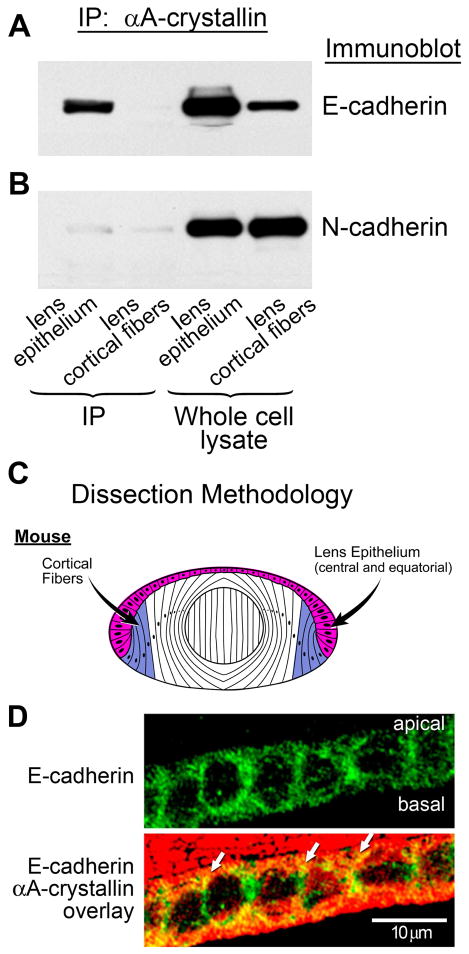
Figure 2.
Association of αA-crystallin with E-cadherin in lens epithelial cells. (A) Co-immunoprecipitation analysis of lens extracts following immunoprecipitation with an antibody to αA-crystallin, and immunoblotting with an antibody to E-cadherin. Note the strong association of αA-crystallin with E-cadherin in the lens epithelium, but no detectable association in lens cortical fibers. (B) Co-immunoprecipitation analysis of lens extracts following immunoprecipitation with an antibody to αA-crystallin and immunoblotting with an antibody to N-cadherin. Note that the association of N-cadherin with αA-crystallin was very low in both lens epithelium and lens cortical fiber cells. Whole cell lysates demonstrate the relative levels of E- and N-cadherin expression in lens epithelium and cortical fibers. (C) Diagram of dissection scheme for obtaining lens epithelial (red) and cortical fiber (blue) cells for these analyses from mouse lenses. In these preparations, the lens epithelial fraction comprised both central and equatorial epithelial cells. (D) Transverse sections of cortical lens fiber cells in adult wild-type mouse lenses were double stained for E-cadherin and αA-crystallin. E-cadherin was detected all along the lateral interfaces of these lens epithelial cells; αA-crystallin co-localized with E-cadherin (white arrows) particularly at the apical borders of these cells.