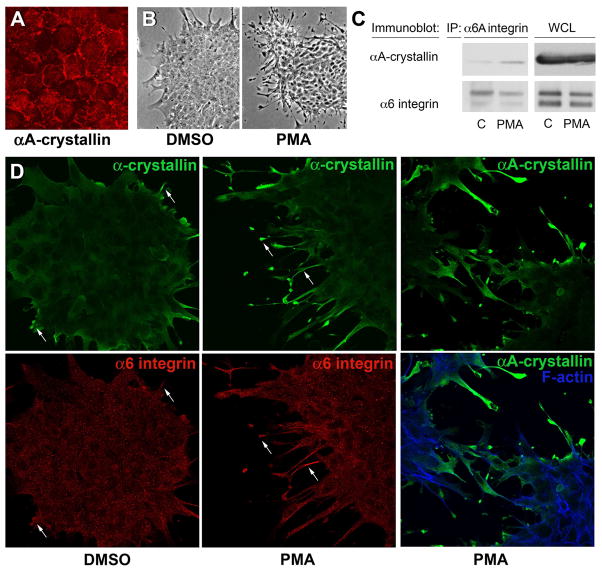
Figure 4.
PMA activation of α6 integrin induces its association with αA-crystallin. (A) Confocal imaging of quail embryo lens epithelial cell cultures immunostained for αA-crystallin (red) showed αA-crystallin localized to cell-cell interfaces, demonstrating that these cells are a good model for studying the mechanism of αA-crystallin association with the plasma membrane. The dependence of αA-crystallin recruitment to α6 integrin on the activation state of this integrin was examined using PMA to activate α6 integrin. Control cultures were treated with DMSO. (B) Efficacy of PMA activation of the α6 integrin receptor was demonstrated by the elaboration of extensive lamellipodial/filopodial processes by the lens epithelial cells along a laminin coated substrate. (C) Recruitment of αA-crystallin to activated α6A integrin signaling complexes was determined following immunoprecipitation of α6 integrin with an antibody to the α6A subunit. Immunoprecipitates were immunoblotted with antibodies to both α6 integrin and αA-crystallin. PMA exposure induced αA-crystallin recruitment to α6 integrin complexes. (D) Co-localization of a-crystallins and α6 integrin in lamellipodial/filopodial processes extended in the presence of PMA was demonstrated by co-immunostaining. Cultures treated with either DMSO or PMA were double-labeled with antibodies to α6 integrin and α-crystallin (αA/αB-crystallin), or singly labeled with antibody to αA-crystallin and double stained with a nuclear marker. Both α-crystallins and α6 integrin were prominently localized to the processes the lens epithelial cells extended on the laminin substrate (arrows).