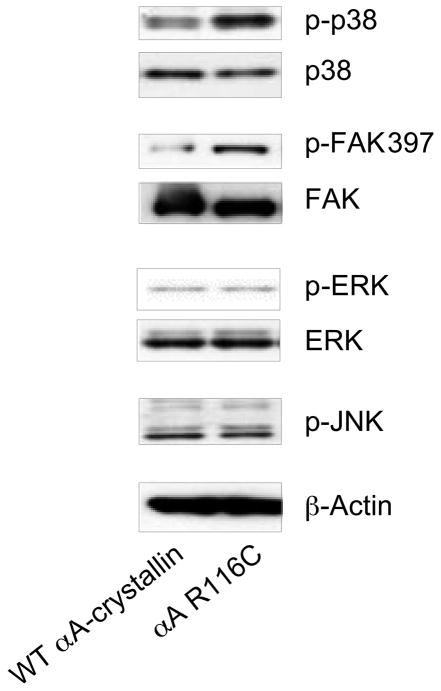
Figure 7.
The αA-crystallin R116C mutant induces activation of p38 and FAK. Extracts of HLE B-3 lens epithelial cells transfected with either wild-type αA-crystallin or the αA-crystallin R116C mutant were examined by immunoblot analysis with antibodies to p-p38, p38, pFAK397, FAK, p-ERK, ERK, p-JNK, and β-actin. Expression of the αA-crystallin R116C mutant in lens epithelial cells induced significant activation of FAK and the stress kinase p38 compared with lens epithelial cells transfected with wild-type αA-crystallin. There was little difference in the activation state of JNK or ERK between αA-crystallin R116C- and WT-transfected lens epithelial cells.