Dear Editor:
The pBi-L plasmid (Clontech, Incorporated, Mountain View, CA) allows dual expression of a gene of interest together with a luciferase marker, and both genes in this vector can be regulated in response to doxycycline or tetracycline. As a result, pBi-L and related vectors are of considerable utility for the generation and study of transgenic animals, and have become increasingly popular for this purpose. With the goal of generating transgenic mice expressing defined thyroid hormone receptor alleles (TRs), we generated a pBi-L vector containing the wild-type TRα1 isoform. Our initial characterizations of this recombinant vector in cultured Hepa 1-6 mouse hepatoma cells confirmed, as anticipated, that both the luciferase gene and the TRα1 gene were expressed in the presence of a tTA transactivator, and that both genes were negatively regulated by doxycycline under these conditions (compare lane 7 with lane 5 in Fig. 1A and lane 3 with lane 1 in Fig. 1B). Unexpectedly expression of both TRα1 and luciferase was also strongly negatively regulated by thyroid hormone (T3) (compare lane 6 with lane 5 in Fig. 1A and lane 2 with lane 1 in Fig. 1B). The negative regulation by T3 could be observed whether the TRα1 was introduced in cis in the pBi-L vector itself (Fig. 1A, lanes 5–8) or was instead expressed in trans from another vector construct (Fig. 1A, lanes 9–12), but was absent in cells lacking TRs (Fig. 1A, lanes 1–4). Similar results were observed when using the TR/J1 isoform (Fig. 1A, lanes 13–20) and in additional cell lines (e.g. human HepG2, Fig. 1B). Notably a fragment of the pBi-L vector created by restriction enzyme digestion and limited to the bidirectional O-Tet-CMV promoter and the luciferase coding region (tetO-Luc) also displayed negative T3 regulation (Fig. 1C; data not shown). In contrast, pUHD10-3, a tet-regulated, but unidirectional expression vector lacking a luciferase marker, did not display this negative T3 regulation (lanes 5–8 in Fig. 1B). Unlike positive T3 response elements, there is no single agreed consensus sequence for a negative T3 response element (e.g. Saatcioglu et al. [2]) and we have not yet mapped the negative response element to a specific sequence within the luciferase gene; we also note that although our data maps one negative T3 response element (nTRE) in pBi-L to the luciferase gene, we cannot exclude the possibility that additional nTREs exist elsewhere in this vector.
FIG. 1.
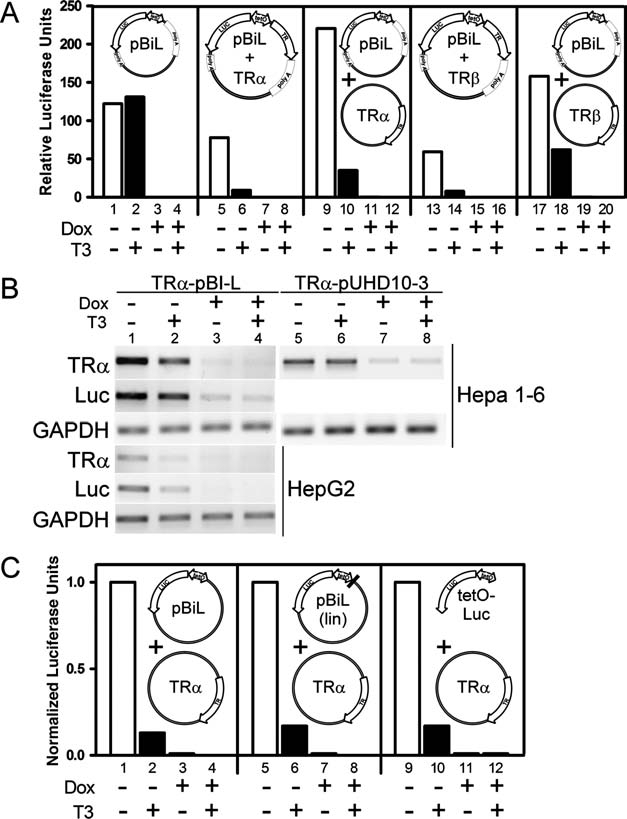
Regulation of pBi-L by doxycycline and thyroid hormone (T3). (A) From left to right, the pBi-L plasmid itself (pBi-L), the same vector with a TRα1 insert, the pBi-L plasmid together with a pSG5-TRα1 expression vector, the pBi-L vector with a TRβ1 insert, or the pBi-L plasmid together with a pSG5-TRβ1 expression vector were transiently transfected into Hepa 1-6 cells using the lipofection procedure previously described (1). Transfections also included a pCH110 β-galactosidase construct as an internal normalization control (1). Cells were maintained at 37°C in Dulbecco-modified Eagles medium containing 10% hormone-depleted fetal bovine serum. After 24 hours the transfection medium was replaced with medium containing doxycycline, T3, or both, as indicated below each panel. After an additional 24 hours the cells were harvested and relative luciferase activity was calculated (1) and is presented. (B) The overall transfection protocol in panel A was repeated using either the TRα1-pBi-L construct described above, a related (TRα-pUHD10-3) construct based on a similar plasmid lacking the luciferase gene (pUHD10-3), and either Hepa 1-6 cells or HepG2 cells as indicated. After harvesting the cells, mRNA was isolated and quantified for levels of TRα1, luciferase, and GAPDH using a reverse transcription PCR protocol (4). (C) Alternatively, the pBi-L vector, a linearized version of the pBi-L vector (lin), or a fragment of the same construct limited to the luciferase-bidirectional promoter region (tetO-Luc) were tested together with the TRa expression vector in the same transfection/luciferase assay as described in panel A.
The powerful T3-mediated down-regulation of pBi-L transcription is likely to introduce significant distortions in the expression patterns of these constructs in different tissues and under different physiological states. We therefore recommend caution in the use of pBi-L and related vectors in studies of thyroid hormone endocrinology. A negative T3 response element has also been identified in vectors possessing a thymidine-kinase promoter-driven luciferase construct, indicating that this may be a general concern when using luciferase as a reporter (3). However, luciferase-based vectors are widely used in cell transfections and many do not appear to display the negative T3 response reported here. Presumably the specific sequence and topology of each vector construct can influence the negative T3 response, indicating that a given vector must be individually evaluated experimentally for its suitability in a particular experiment.
Acknowledgments
This work was supported by United States Public Health Service/National Institutes of Health grants RO1DK53528, 5U42RR014905, and 1K26RR024037.
References
- 1.Chan IH. Privalsky ML. Thyroid hormone receptors mutated in liver cancer function as distorted antimorphs. Oncogene. 2006;25:3576–3588. doi: 10.1038/sj.onc.1209389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saatcioglu F. Claret FX. Karin M. Negative regulation by nuclear receptors. Semin Cancer Biol. 1994;5:347–359. [PubMed] [Google Scholar]
- 3.Maia AL. Harney JW. Larsen PR. Is there a negative TRE in the luciferase reporter cDNA? Thyroid. 1996;6:325–328. doi: 10.1089/thy.1996.6.325. [DOI] [PubMed] [Google Scholar]
- 4.Goodson ML. Jonas BA. Privalsky ML. Alternative mRNA splicing of SMRT creates functional diversity by generating corepressor isoforms with different affinities for different nuclear receptors. J Biol Chem. 2005;280:7493–7503. doi: 10.1074/jbc.M411514200. [DOI] [PMC free article] [PubMed] [Google Scholar]


