Figure 3. TLR-activation by cell culture-derived recombinant infectious HCV (HCVcc).
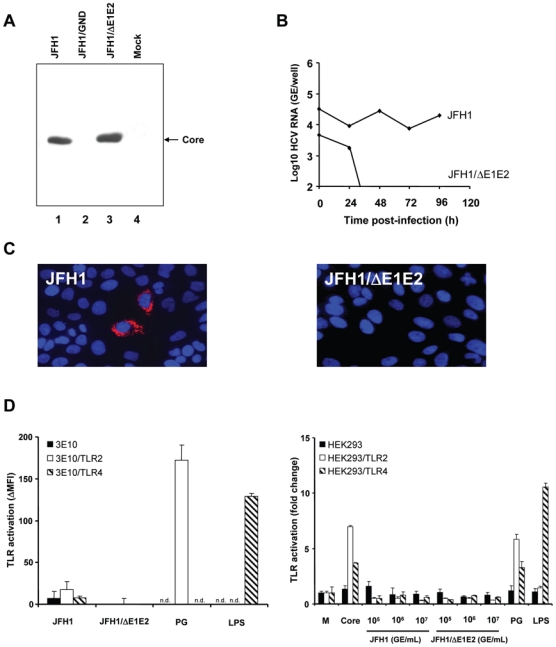
(A) Production of HCVcc and control supernatants by transfection of Huh7.5 cells with JFH1, JFH1/GND and JFH1/ΔE1E2 RNA. Huh7.5 cells were lysed 96 h after electroporation of full-length JFH1, JFH1/GND containing a mutation in the RNA polymerase or envelope protein-deficient JFH1/ΔE1E2 HCV RNA. Proteins were separated by SDS-PAGE, followed by immunoblotting with anti-core monoclonal antibody. (B) HCVcc-infection of Huh7.5 cells assessed by RT-PCR of intracellular HCV RNA. Huh7.5 cells were incubated for 3 h at 37°C with HCVcc at 107 genome equivalents/mL (GE/mL, JFH1 or JFH1/ΔE1E2) and subsequently incubated for 24 h, 48 h, 72 h and 96 h at 37°C with Huh7.5 medium. Intracellular HCV RNA was quantified. (C) Analysis of HCVcc-infection of Huh7.5 cells by immunofluorescence. Huh7.5 cells were incubated for 3 h at 37°C with HCVcc at 107 GE/mL (JFH1 or JFH1/ΔE1E2) and subsequently incubated for 48 h at 37°C with Huh7.5 medium. HCV core protein expression was detected using an anti-core monoclonal antibody and a Cy3-conjugated anti-mouse IgG antibody (red fluorescence). Nuclear staining was performed using DAPI (4′, 6-diamino-2-phenylindole; blue fluorescence). (D) TLR-activation by HCVcc - as produced and characterized in panels A-C - in transfected CHO and HEK293 cells. CHO cells were incubated with HCVcc at 107 GE/mL and HEK293 with HCVcc at 105, 106 and 107 GE/mL (corresponding to approximately 150 pg/mL to 33 ng/mL core protein) for 18 h at 37°C. Incubation of cells with complete medium (M), PG (10 μg/mL), LPS (1 μg/mL) or recombinant core protein (10 μg/mL) served as negative and positive controls, respectively. TLR-activation was determined as described in legend of Fig. 2. n.d.: not determined.
