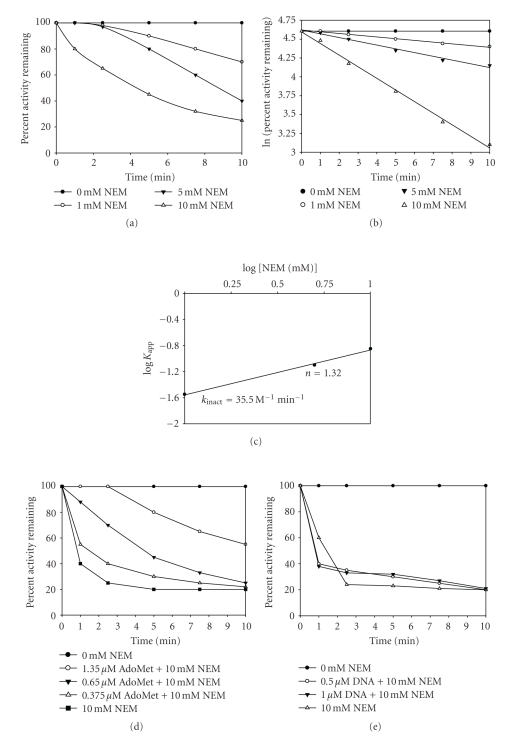
Figure 7.
Kinetics of inactivation of M. EcoP1I by N-ethylmaleimide. M. EcoP1I (6.6 μM) was incubated at 25°C in 10 mM sodium phosphate (pH 6.8) containing 0–10 mM NEM. At the indicated times, aliquots were withdrawn and assayed for DNA MTase activity using filter binding assay as described Section 2. (a) Time course of inactivation plot was constructed. Control incubations gave no change in activity. (b) The pseudo-first-order rate constants (K app) were calculated from the slopes of the inactivation plot for each concentration of NEM used. (c) Pseudo-first-order plot. The apparent first-order rate constants (K app) were plotted against log[NEM]. The y intercept gives the rate of inactivation of the enzyme (K inact). The slope of the line gives the number of cysteine residues modified (n). Values are averages of triplicate determinations. (d) M.EcoP1I (6.6 μM) was preincubated with 0.375 μM, 0.65 μM, and 1.35 μM [3H-methyl] AdoMet for 10 minutes, followed by addition of 10 mM NEM. Methylation activity of all the samples was analyzed under standard reaction conditions so that the final concentration of AdoMet was the same. (e) M.EcoP1I (6.6 μM) was preincubated with 0.5 μM and 1.0 μM duplex I for 10 minutes, followed by addition of 10 mM NEM. Methylation activities of all samples were analyzed under standard reaction conditions.