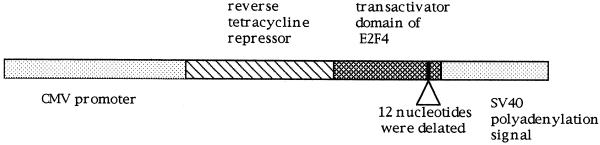
Figure 1.
The structure of the novel transactivator. The transactivator domain (amino acids 297–413) of E2F4 was fused to reverse type tetracycline repressor. In order to prevent the RB family proteins from binding to E2F4, four residues (amino acids 404–407) of the pocket protein binding domain of E2F4 were deleted. This transactivator is driven by the CMV promoter (prTE4d38).