Abstract
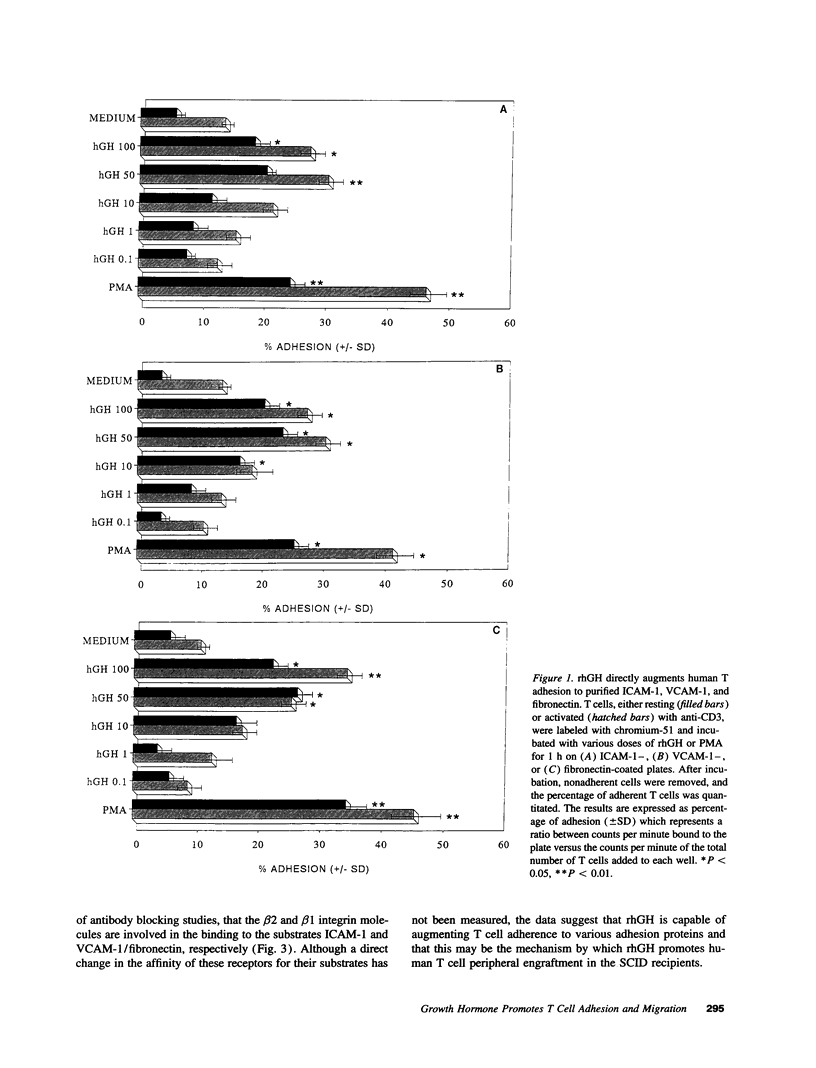
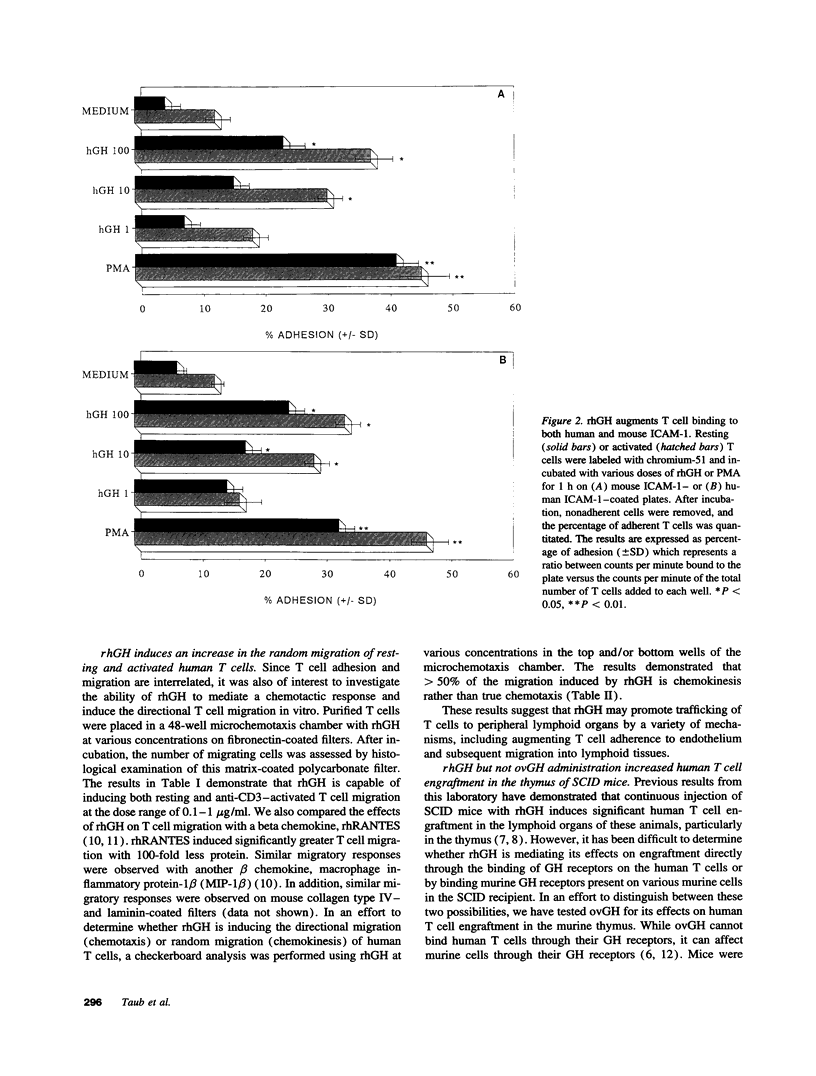
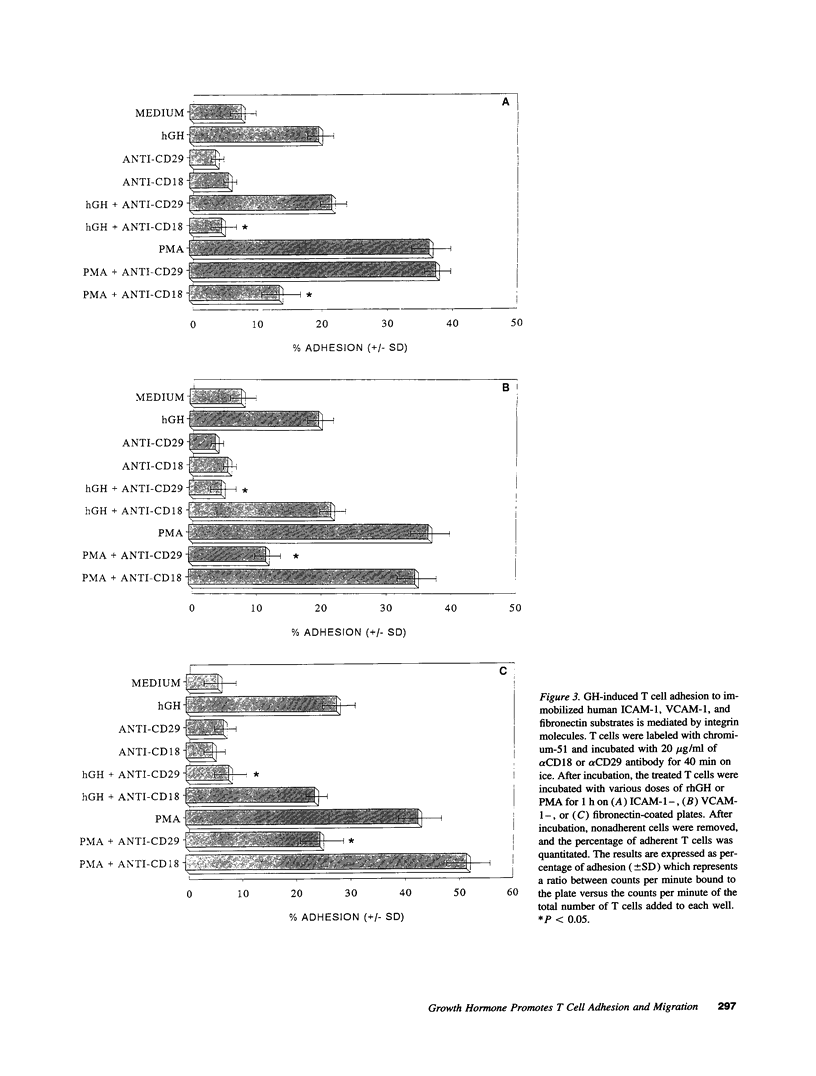
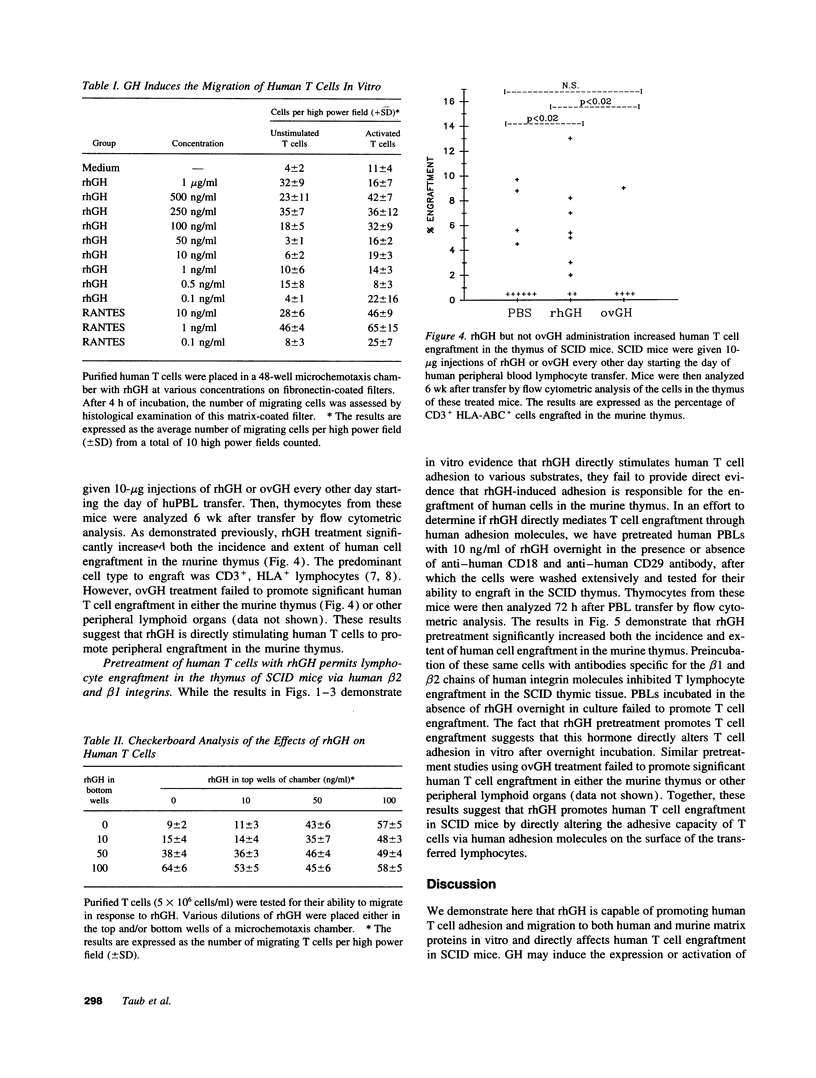
Recombinant human growth hormone (rhGH) promotes human T cell engraftment in mice with severe combined immunodeficiency, suggesting that rhGH may have effects on T cell adhesion and migration in vivo. The ability of rhGH to directly affect the adhesion capacity of human T cells to a variety of human or murine adhesion molecules and extracellular matrix proteins was examined. rhGH induced significant human T cell adherence to both human and murine substrates via either beta 1 or beta 2 integrin molecules. rhGH was capable of inducing significant migration of resting and activated human T cells and their subsets. Most of the migratory response to rhGH was chemokinetic rather than chemotactic. In vivo engraftment studies in severe combined immunodeficiency mice receiving human T cells revealed that treatment with rhGH resulted in improved thymic engraftment, whereas treatment with non-human-reactive ovine GH demonstrated no significant effects. These data demonstrate that rhGH directly augments human T cell trafficking to peripheral murine lymphoid tissues. rhGH appears to be capable of directly altering the adhesive and migratory capacity of human T cells to molecules of either murine or human origin. Therefore, GH may, under either isogeneic or xenogeneic conditions, play a role in normal lymphocyte recirculation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K. W. Growth hormone, lymphocytes and macrophages. Biochem Pharmacol. 1989 Mar 1;38(5):705–713. doi: 10.1016/0006-2952(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Bennett M., Anver M. R., Baseler M., Longo D. L. Human-mouse lymphoid chimeras: host-vs.-graft and graft-vs.-host reactions. Eur J Immunol. 1992 Jun;22(6):1421–1427. doi: 10.1002/eji.1830220614. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Anver M., Frazier M., Longo D. L. Recombinant human growth hormone promotes human lymphocyte engraftment in immunodeficient mice and results in an increased incidence of human Epstein Barr virus-induced B-cell lymphoma. Brain Behav Immun. 1992 Dec;6(4):355–364. doi: 10.1016/0889-1591(92)90034-l. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Longo D. L. Differential effects of growth hormone and prolactin on murine T cell development and function. J Exp Med. 1993 Jul 1;178(1):231–236. doi: 10.1084/jem.178.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Longo D. L. Human growth hormone promotes engraftment of murine or human T cells in severe combined immunodeficient mice. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4481–4485. doi: 10.1073/pnas.89.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Longo D. L. Role of neuroendocrine hormones in murine T cell development. Growth hormone exerts thymopoietic effects in vivo. J Immunol. 1992 Dec 15;149(12):3851–3857. [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Rui H., Djeu J. Y., Evans G. A., Kelly P. A., Farrar W. L. Prolactin receptor triggering. Evidence for rapid tyrosine kinase activation. J Biol Chem. 1992 Nov 25;267(33):24076–24081. [PubMed] [Google Scholar]
- Snow E. C., Feldbush T. L., Oaks J. A. The effect of growth hormone and insulin upon MLC responses and the generation of cytotoxic lymphocytes. J Immunol. 1981 Jan;126(1):161–164. [PubMed] [Google Scholar]
- Taub D. D., Conlon K., Lloyd A. R., Oppenheim J. J., Kelvin D. J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993 Apr 16;260(5106):355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Wiedermann C. J., Reinisch N., Braunsteiner H. Stimulation of monocyte chemotaxis by human growth hormone and its deactivation by somatostatin. Blood. 1993 Aug 1;82(3):954–960. [PubMed] [Google Scholar]
- van Buul-Offers S., Van den Brande J. L. The growth of different organs of normal and dwarfed Snell mice, before and during growth hormone therapy. Acta Endocrinol (Copenh) 1981 Jan;96(1):46–58. doi: 10.1530/acta.0.0960046. [DOI] [PubMed] [Google Scholar]










