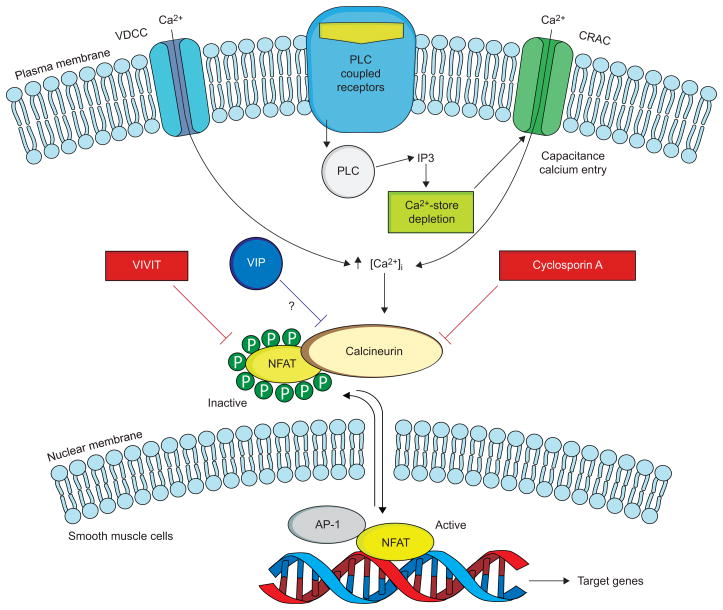
FIGURE 1.
The calcineurin–NFAT (nuclear factor of activated T-cells) pathway and its possible regulation by vasoactive intestinal peptide (VIP). In resting cells, NFAT resides in the cytoplasm, where it is maintained in a phosphorylated, inactive state. Stimulation of cell-surface receptors coupled to phospholipase C (PLC) results in calcium mobilisation, initially from intracellular stores, followed by influx of Ca2+ through specialised voltage-dependent Ca2+ channels (VDCC) and Ca2+ release-activated Ca2+ channels (CRAC). Upon activation by Ca2+, the phosphatase calcineurin dephosphorylates NFAT, triggering its activation and nuclear translocation. In the nucleus, NFAT interacts with other transcription factors, including activator protein (AP)-1, to stimulate gene expression. When NFAT is re-phosphorylated it is exported back to the cytoplasm. The immunosuppressive drug cyclosporin A inhibits calcineurin interactions with all its substrates, whereas VIVIT selectively inhibits NFAT activation. VIP appears to inhibit this pathway, but its mechanism of action has yet to be determined. IP3: inositol-1,4,5-triphosphate. Modified from [26].