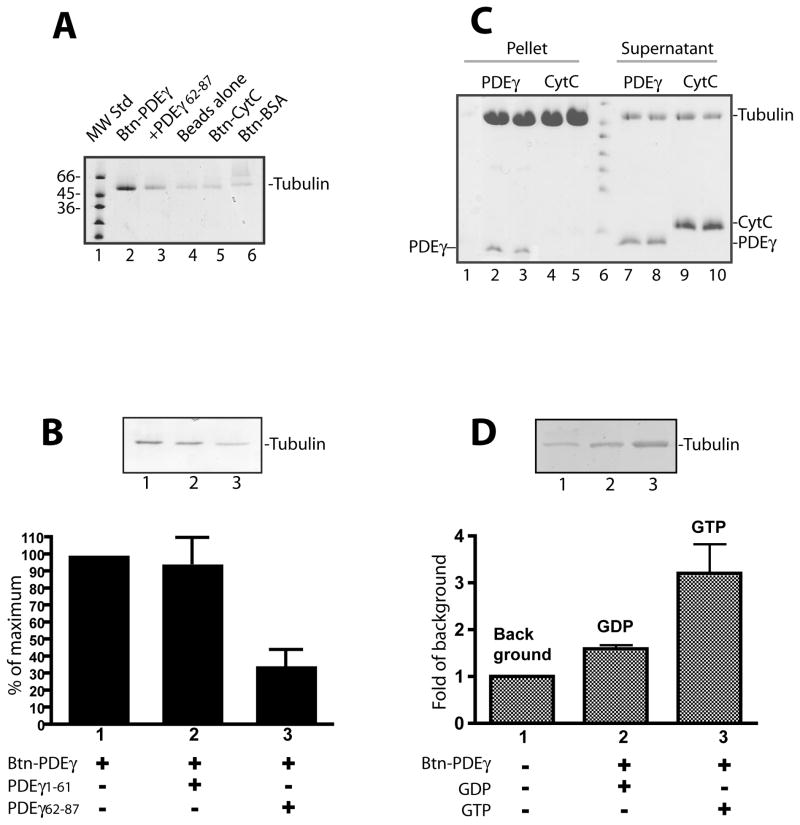
Figure 2. Specific PDEγ:tubulin interaction revealed by assays using pure proteins.
Streptavidin beads pull down assays (A, B, D) or microtubule co-sedimentation assay (C) were performed using pure tubulin proteins.
A. Btn-PDEγ pulled down microtubules specifically (lane 2) as compared with various controls including the beads with no Btn-PDEγ bound (lane 4), the beads with Btn-CytC bound (lane 5), and the beads with Btn-BSA bound (lane 6). In lane 3, the PDEγ C-terminal peptide PDEγ62-87 in a 20 fold molar excess over Btn-PDEγ was added. B. Microtubules pull down by Btn-PDEγ on the streptavidin beads (lane 1) was reduced in the presence of PDEγ62-87 (lane 2) but not PDEγ1-61 (lane 3) in a 20 fold molar excess. Pull down was quantitated as percent of the Coomassie staining in the absence of PDEγ peptides (lane 1). Error bar is a standard deviation from 3 separated experiments. Student’s t-tests indicate that the difference between bars 2 and 1 is insignificant (p>0.5); whereas the difference between bars 3 and 1 is very significant (p<0.1). C. Co-sedimented proteins are shown in lanes 2–5; the supernatants of the corresponding conditions are shown in lanes 7–10. Each condition is duplicates except lane 1, which is the control of PDEγ pellet from the reaction with no microtubules. D. GTP dependence of tubulin pull down by Btn-PDEγ-beads. Tubulin (9 μg) was incubated with beads alone (lane 1) or beads prebound with 1 μg Btn-PDEγ for 1 hr at room temperature in the buffer containing 20 mM HEPES, pH 7.4, 120 mM NaCl, and 5 mM MgCl2, in the presence of 2 mM GDP (lane 2) or 2 mM GTP (lane 3). Pull down was quantitated as fold of the Coomassie staining in the control (lane 1). Error bar is a standard deviation of 2 separate experiments. The difference between the GDP condition and the GTP condition is significance (p= 0.207).