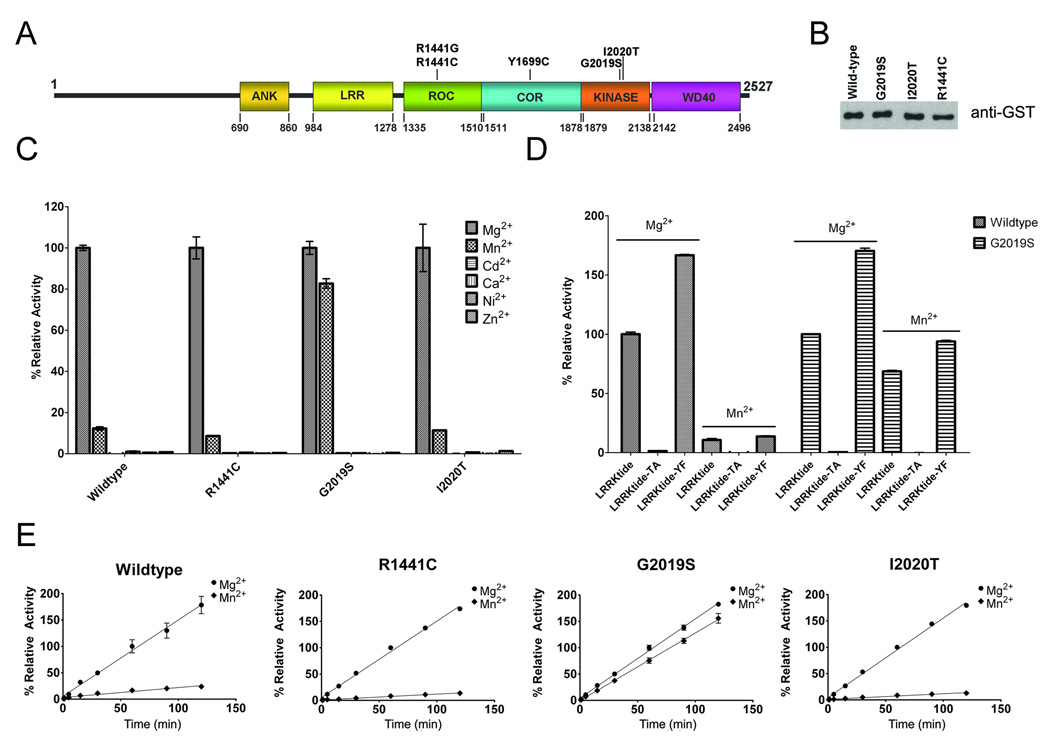
Figure 1. Characterization of recombinant LRRK2 kinase activity.
(A) Schematic of LRRK2 showing the major domains [ankyrin-like (ANK), Leu-rich repeat (LRR), Ras-in-complex (ROC), C-terminal of RAS (COR)] and the position of the mutations that are considered definitely pathogenic. (B) Western blot with anti-GST antibody showing equal amounts of glutathione affinity-purified recombinant WT and mutant (G2019S, I2020T and R1441C) GST-LRRK2 full-length proteins. (C) Relative kinase activity of WT, R1441C, G2019S, and I2020T LRRK2 using 200 µM ATP, 400 µM LRRKtide and several individual divalent cations (Mg2+, Mn2+, Cd2+, Ca2+, Ni2+, Zn2+) at 5 mM. The data was standardized so that the phosphorylation reaction of LRRKtide with Mg2+ for each LRRK2 variant was normalized to 100%. (D) Comparative assessment of the ability of WT and G2019S LRRK2 to phosphorylate LRRKtide, LRRKtide-TA or LRRKtide-YF (300 µM each) in the presence of 200 µM ATP and either 5 mM Mg2+ or Mn2+. (E) Assay demonstrating that the time-course of LRRK2 kinase activity was linear over 120 min using 200 µM ATP, 400 µM LRRKtide and either 5 mM Mg2+ or Mn2+. For each LRRK2 variant, the activity was standardized as 100% for kinase reactions in 5 mM Mg2+ at 60 minutes. The error bars represent standard error of the mean.