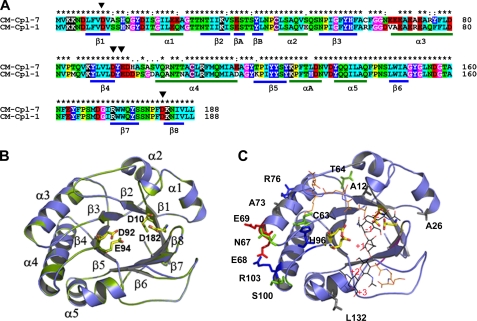
FIGURE 4.
Homology model of the Cpl-7 catalytic module. A, sequence alignments of Cpl-7 and Cpl-1 CMs. Color code is as used in Fig. 2. The position of β-strands and α-helices in the CM of Cpl-1 is shown. Black triangles indicate the catalytic residues (Asp10, Asp92, Glu94, and Asp182). B, superimposition of the template (green) and model (blue) structures. Catalytic residues are in stick representation (Cpl-7, yellow; Cpl-1, green). C, superimposition of the coordinates for the Cpl-1-(2S5P)3 complex (10) with the computational model of Cpl-7 CM. Nonconserved residues together with substitutions relevant for substrate binding are shown in stick representation (red, acidic; blue, basic; green, polar uncharged; and gray, hydrophobic). (2S5P)3 stands for (GlcNAc-MurNAc-(l-Ala-d-isoGln-l-Lys-d-Ala-d-Ala)3); carbon atoms are in black (sugar chain) or orange (peptide stem).