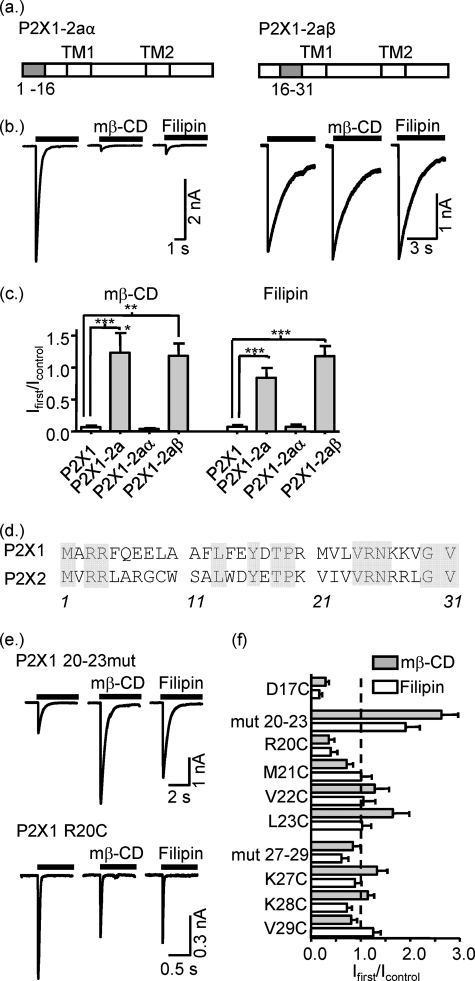
FIGURE 5.
Identification of the regions in the amino terminus of the P2X1 receptor contributed to lipid raft disruption sensitivity. a, schematic representation of P2X1-2aα and P2X1-2aβ chimera constructs where 1–16 and 16–31 residues from the P2X1 receptor were swapped for P2X2 ones, respectively. b, representative currents mediated by P2X1-2aα and P2X1-2aβ chimeric receptors in HEK293 cells in control conditions and after treatment with mβ-CD or filipin. Currents were induced by application of ATP (100 μm) for 5 s. c, summary of the effects of mβ-Cd and filipin treatment on currents mediated by P2X1, P2X1-2a, P2X1-2aα, and P2X1-2aβ chimeric receptors as fraction of the response to ATP in nontreated cells (n = 6–14). d, amino acid sequences of P2X1 and P2X2 intracellular amino termini. Conserved amino acids are highlighted gray. e, representative currents mediated by P2X1(20–23) and P2X1 R20C mutants evoked by application of ATP in control conditions and after treatment with mβ-CD or filipin. f, summary of the effects of mβ-CD and filipin treatment on currents mediated by P2X1 mutants (n = 5–10). All of them were significantly different from the P2X1 receptor wild type (not shown on figure). *, p < 0.05; **, p < 0.01; ***, p < 0.001.