FIGURE 1.
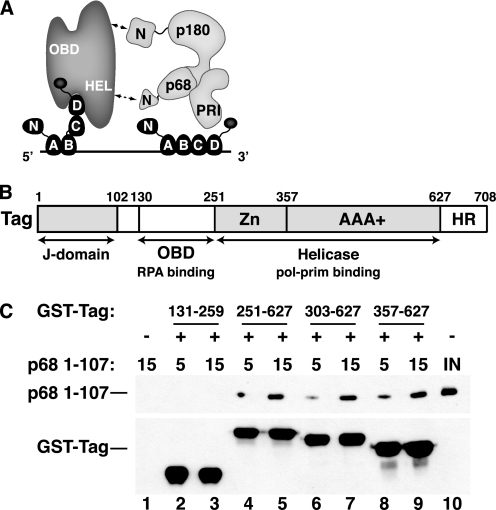
Tag 357–627 is sufficient to bind to pol-prim p68 1–107. A, a molecular handoff model for SV40 primosome activity on RPA-coated ssDNA. The four ssDNA-binding domains (A–D) of RPA (dark gray) occlude up to 30 nucleotides of ssDNA (straight line). Flexible linkers (wavy lines) join the N-terminal domain of RPA70 and the C-terminal domain of RPA32 to the RPA/ssDNA. Tag contacts with RPA32C and RPA70AB remodel it into a more compact, lower affinity ssDNA-binding mode and stabilize it as a ternary complex (22, 23, 35), transiently exposing the template ssDNA. pol-prim (light gray) contacts the Tag helicase domains (HEL) through p68N (36), the N terminus of p180 DNA polymerase, and unknown surfaces of primase p58/p48 (PRI) (27, 29, 30). The ensemble of these interactions is proposed to position primase on the exposed template to synthesize an RNA primer (not shown). B, domain architecture of SV40 Tag. The DnaJ chaperone domain (72), SV40 OBD (73), and helicase domain (42, 43) are depicted. The structure of the host-range (HR) domain is not known (74). C, GST-tagged Tag fragments 131–259 (lanes 2 and 3), 251–627 (lanes 4 and 5), 303–627 (lanes 6 and 7), or 357–627 (lanes 8 and 9) adsorbed to glutathione beads were incubated with increasing amounts of His-tagged p68 1–107 as indicated. Proteins bound to the beads were separated by SDS-PAGE and visualized by Western blotting with anti-His antibody (top) or anti-GST antibody (bottom). Glutathione beads lacking GST-Tag protein (lane 1) are shown as negative control. Lane 10 shows 200 ng of input p68 1–107.