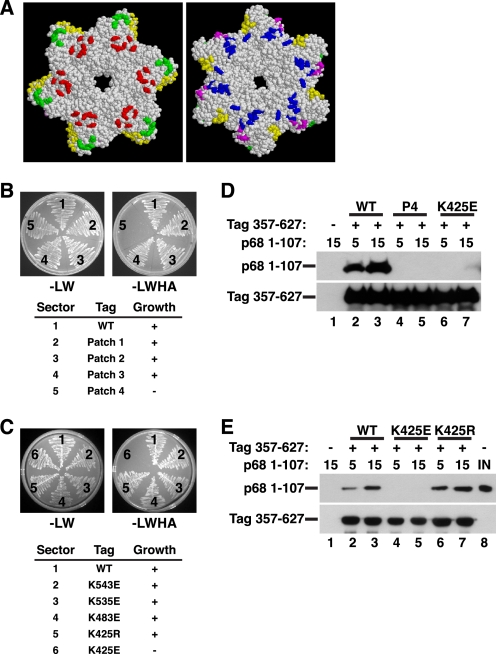
FIGURE 2.
Structure-guided mutagenesis of Tag surface residues to map the p68N-docking site. A, diagram of conserved patches of charged surface residues of the Tag hexamer (residues 266–627) (45) (reprinted with permission). In the left view, the six zinc subdomains face the reader; the right view is rotated 180° so that the AAA+ subdomains face the reader. Green, patch 1; yellow, patch 2; red, patch 3; blue, patch 4; magenta, patch 5. Patch mutants (B) and single residue substitutions of pGADT7-fused Tag (C) were screened in yeast two-hybrid assays for interaction with pGBKT7-fused p68 1–107. The numbered sectors are identified in the tables below. Left panel, control plate -Leu -Trp; right panel, selective plate -Leu -Trp -His -Ade. D, glutathione beads alone (lane 1) or adsorbed to WT (lanes 2 and 3), patch 4 (P4) mutant (lanes 4 and 5), or K425E GST-Tag 357–627 (lanes 6 and 7) were incubated with 5 or 15 μg of His-tagged p68 1–107 as indicated. Bound proteins were analyzed by Western blotting with anti-His (top) or anti-GST antibody (bottom). E, glutathione beads alone (lane 1) or adsorbed to GST-Tag 357–627 WT (lanes 2 and 3), K425E (lanes 3 and 4), or K425R (lanes 6 and 7) were incubated with 5 or 15 μg of p68 1–107 as indicated. Bound proteins were analyzed by Western blotting with anti-His (top) or anti-GST antibody (bottom). Lane 8 shows 200 ng of input p68 1–107.