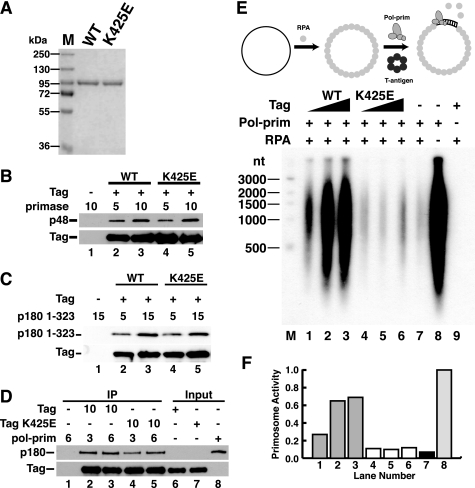
FIGURE 3.
K425E Tag binds to primase and p180 pol-prim but lacks primosome activity. A, purified WT and K425E Tag were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. M, protein size markers. B and C, Pab101 beads alone (lane 1) or bound to WT (lanes 2 and 3) or K425E Tag (lanes 4 and 5) were incubated with increasing amounts of primase dimer (B) or His-p180 1–323 (C) as indicated. Bound proteins were detected by SDS-PAGE and immunoblotting with anti-p48, anti-His (catalog no. A00186, Genscript), or Pab101 against Tag. D, anti-Tag beads alone (lane 1) or adsorbed to 10 μg of WT (lanes 2 and 3) or K425E Tag (lanes 4 and 5) were incubated with 3 or 6 μg of pol-prim as indicated. Bound proteins were analyzed by Western blotting with anti-Tag and anti-p180 antibody as indicated. Input, 100 ng. E and F, primosome activity of 200, 400, or 600 ng of Tag WT (lanes 1–3) or K425E (lanes 4–6) was assayed on 100 ng of ssDNA precoated with 1 μg of RPA in the presence of 600 ng of pol-prim. Control reactions lacked Tag (lane 7), Tag and RPA (lane 8), or pol-prim (lane 9). Reaction products were analyzed by alkaline electrophoresis and visualized by autoradiography (E). DNA size markers are shown (M). F, reaction products were quantified; signal in the negative control reaction (lane 9) was subtracted from that in lanes 1–8. Incorporation in lanes 1–7 is expressed as a fraction of that in lane 8.