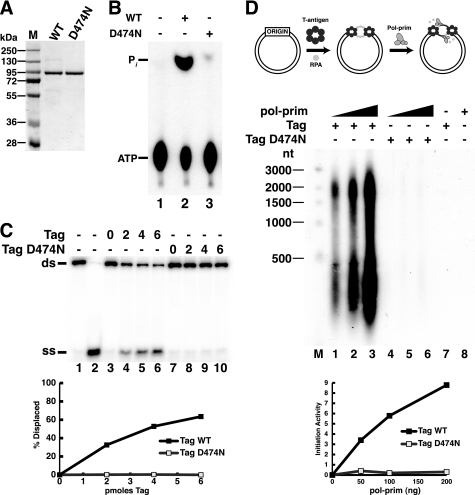
FIGURE 5.
A single residue substitution in the Walker B motif of Tag abolishes ATPase/helicase activity and initiation of SV40 replication. A, purified WT and D474N Tag were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. M, protein size markers. B, ATPase reactions were carried out without Tag (lane 1) or with 1 μg of Tag WT (lane 2) or D474N (lane 3). The reaction products were separated by ascending thin layer chromatography and visualized by autoradiography. C, to assess helicase activity, 10 fmol of DNA substrate was incubated without Tag (lanes 3 and 7) or with increasing amounts (2, 4, or 6 pmol) of WT (lanes 4–6) or D474N (lanes 8–10) Tag. Lanes 1 and 2 contain DNA substrate or boiled substrate alone. D, SV40 initiation activity of 600 ng Tag WT (lanes 1–3) or D474N (lanes 4–6) was assayed in monopolymerase reactions with 50, 100, or 200 ng of pol-prim. Radiolabeled DNA products were visualized by alkaline agarose electrophoresis and autoradiography. Products of control reactions without pol-prim (lane 7) or Tag (lane 8) are shown as indicated (−). End-labeled DNA fragments of the indicated sizes are shown at the left (M). Reaction products were quantified; background (lanes 7 and 8) was subtracted from incorporation in lanes 1–6 (lower panel). ss, single strand; ds, double strand.