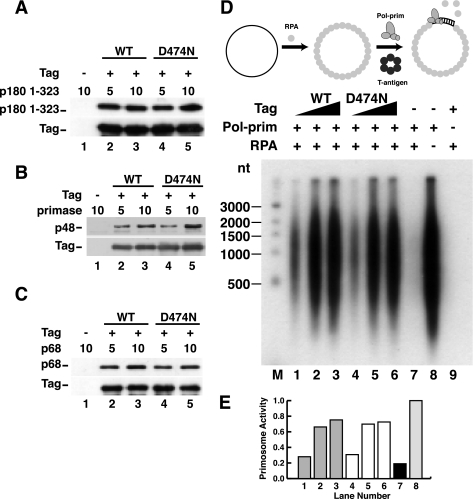
FIGURE 6.
ATPase activity of Tag is not required for pol-prim binding or primosome activity on RPA-coated ssDNA. A–C, purified Tag WT or D474N bound to Pab101-coupled Sepharose beads was incubated with increasing amounts of His-p180 1–323 (A), primase dimer (B), or His-p68 (C) as indicated (in μg). Proteins bound to the beads were separated by SDS-PAGE and visualized by Western blotting with anti-His (catalog no. A00186, Genscript) for p180, anti-p48 or anti-His (catalog no. 9801, Abcam) for p68, and Pab101 against Tag. D, primosome activity of 200, 400, or 600 ng of Tag WT (lanes 1–3) or D474N (lanes 4–6) was assayed on 100 ng of ssDNA precoated with 1 μg of RPA in the presence of 600 ng of pol-prim. Control reactions lacking Tag (lane 7), Tag and RPA (lane 8), or pol-prim (lane 9) are indicated. Reaction products were analyzed by alkaline electrophoresis and autoradiography. DNA size markers are shown (M). E, reaction products were quantified; signal in the negative control reaction (lane 9) was subtracted from that in lanes 1–8. Incorporation in lanes 1–7 is expressed as a fraction of that in lane 8.