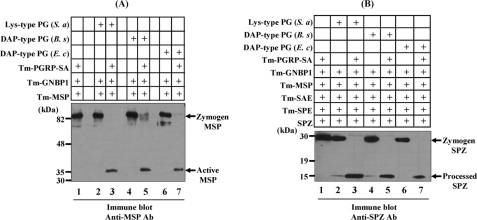
FIGURE 2.
In vitro reconstitution experiments for the activation of pro-MSP and processing of pro-Spätzle by polymeric DAP-type PG. A, mixture of purified Tenebrio PGRP-SA, GNBP1, and MSP zymogen in the presence of S. aureus Lys-type PG (lanes 2 and 3), B. subtilis polymeric DAP-type PG (lanes 4 and 5), and E. coli DAP-type PG (lanes 6 and 7) were incubated for 60 min and then analyzed by Western blotting with an anti-MSP antibody. The 82-kDa pro-MSP and the 35-kDa activated MSP are indicated with arrows. In the absence of PGRP-SA, pro-MSP was not cleaved (lanes 2, 4, and 6). B, mixture of Tenebrio PGRP-SA, GNBP1, MSP, SAE, SPE zymogens, and pro-Spätzle (SPZ) in the presence of S. aureus Lys-type PG (lanes 2 and 3), B. subtilis polymeric DAP-type PG (lanes 4 and 5), and E. coli DAP-type PG (lanes 6 and 7) were incubated for 60 min and then analyzed by Western blotting with an affinity-purified anti-SPZ antibody. The 30-kDa pro-SPZ and the 12-kDa processed SPZ are indicated with arrows. As a control, when eight components, such as Lys-type PG·PGRP-SA·GNBP1·MSP·SAE·SPE·Spätzle, were incubated together, the cleaved 12-kDa SPZ was generated (lane 3). In the absence of PGRP-SA, pro-SPZ was not converted to the processed SPZ (lanes 2, 4, and 6). Tm, T. molitor; Ab, antibody; S. a, S. aureus; B. s, B. subtilis; E. c, E. coli.