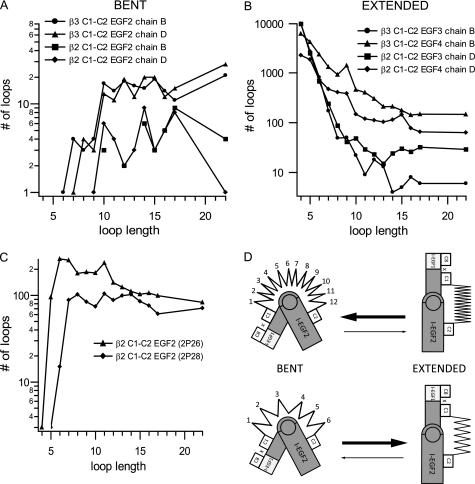
FIGURE 10.
Regulation of integrin conformation by an entropic spring in the β-knee. A–C, as a surrogate for loop entropy, the number of different loops in the Protein Data Bank that could span C1 and C2 in different integrin conformational states was estimated as a function of loop length. A, bent conformation, sampled with the C1-C2 loop of I-EGF2 of two independent αIIbβ3 molecules (chains B and D of 3FCS) and αXβ2 molecules (chains B and D of 3K6S). B, extended conformation, sampled with the C1-C2 loops of I-EGF3 and I-EGF4 of αIIbβ3 and αXβ2. C, relaxed conformation, sampled with the C1-C2 loop of I-EGF2 of β2-leg fragment structures 2P26 and 2P28. D, schematic of an entropic spring in the β-knee. A large number of residues (spring coils) pushes the conformation toward the bent state. A smaller number of residues (spring coils) pulls the conformation toward the extended state.