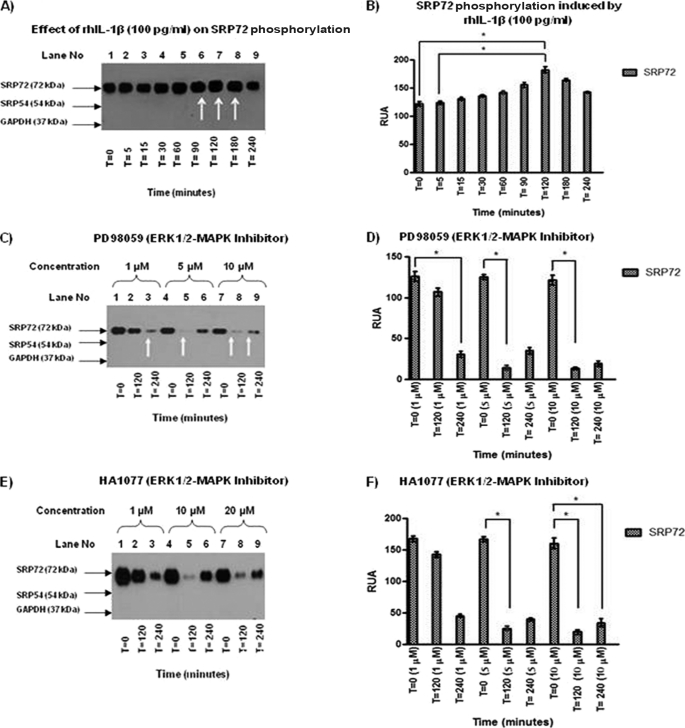
FIGURE 3.
A, IP of Jurkat cell lysates treated with rhIL-1β was carried out using anti-human SRP19 antibody because this is the only antibody able to immunoprecipitate the SRP complex. An anti-phosphoserine antibody was used to identify phosphorylated proteins. An increase of intensity in the SRP72 band at 90, 120, and 180 min (lanes 6–8) was found. B, results were analyzed and RUA illustrated with significant results at 120 versus 0 and versus 5 min, respectively, with p < 0.05. C, effect of ERK1/2 inhibitor PD98059 (1, 5, and 10 μm) was evaluated in SRP72-immunoprecipitated cell lysates. WB using anti-phosphoserine antibody showed a decreased SRP72 band when used as the inhibitor at concentrations of 1 μm at 240 min, 5 μm at 120 min, and 10 μm at 120 and 240 min (lanes 3, 5, 8, and 9). D, results were analyzed and RUA illustrated, finding significant results at 1 μm at 240 versus 0, 5 μm at 120 versus 0, and 10 μm at 120 versus 0 min. E, SRP72 immunoprecipitated from Jurkat cell lysate with anti-human SRP19 antibody was done to evaluate the effect of HA1077 ERK/12 inhibitor. WB using anti-phosphoserine antibody was done to identify phosphorylation status. We found a decreased intensity of SRP72 at concentration of 1 μm (120 min) and 10 and 20 μm (120 min). This finding was interesting because it suggests re-phosphorylation phenomena. F, RUA was illustrated, obtaining significant results at 5 μm at time 0 versus 120 min and 10 μm at 120 versus 0 and 240 versus 0 min (p < 0.05). * indicates p < 0.05. The experiments were repeated three times.