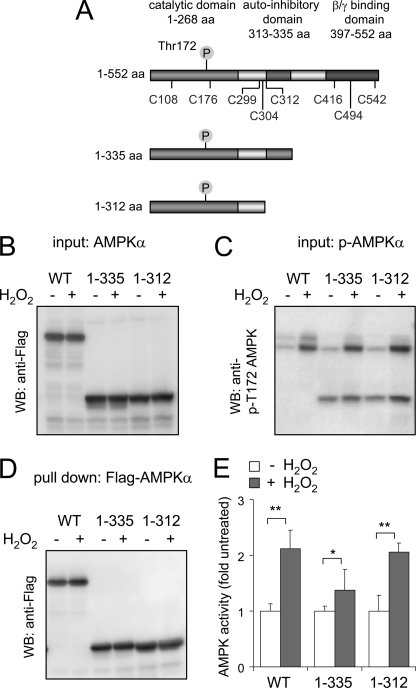
FIGURE 5.
A, human AMPK. The AMPKα subunit with the location of cysteines, phosphorylation site Thr172, catalytic domain, AID, and βγ binding domain and C-terminal truncation sites of AMPKα1 as used in the experiments is shown. B–D, Western blots (WB) of transiently expressed FLAG-AMPKα WT, AMPK 1–335, or AMPK 1–312 obtained from HEK 293 cells treated with H2O2 (0 or 250 μm) for 15 min. B and C show the level of total AMPK by probing the membrane with anti-FLAG antibodies or with antibodies to phospho-Thr172, whereas D shows the amount of FLAG-AMPKα or AMPKα truncation mutants obtained after pull-down with anti-FLAG-agarose. E, FLAG-tagged AMPKα (WT or truncation mutants) was subjected to pull-down, and then AMPK activity was determined using a radiometric assay with SAMS peptide as a substrate. Shown is the mean ± S.D. (error bars), n = 3. *, p < 0.05; **, p < 0.01 compared with untreated. aa, amino acids.