Abstract
Estrogen binding protein activities were determined in the cytosol from adult male Buffalo rat liver and Morris hepatoma 7777. Estrogen receptors were prepared using the protamine sulfate precipitation technique of Chamness. The ability of various unlabeled steroids competing with [3H]estradiol was examined to establish the binding specificity. Estradiol binding in Morris hepatoma 7777 cytosol was greatly decreased compared with that present in hepatic cytosol prepared from normal rat liver. The receptor concentration expressed as femtomoles per milligram of cytoplasmic protein was 31.1 ± 2.9 SD for normal rat liver and 0.41 ± 0.88 SD for the hepatoma. Gel filtration chromatography revealed the presence of an estrogen binder in hepatoma cytosol which was not present in either normal liver or in the protamine sulfate precipitates of hepatoma cytosol. The molecular weight, binding specificity, and precipitation of this protein by specific antiserum suggests that it is α-fetoprotein.
The liver is a steroid-responsive organ both in male and female animals. Moreover, in both sexes many biochemical events occur that are dependent, at least in part, upon steroid hormone action. These include the transport of hydrophobic materials by various plasma proteins synthesized by the liver, such as sex steroid-binding globulin (1), the production of important circulating substances such as renin substrate (2,3), and the production and secretion of various circulating proteins such as ceruloplasmin (4).
After the original discovery of estrogen receptor in the liver (5–12), many attempts have been made to define a possible role for steroid hormones; particularly estrogens, in the pathogenesis of various hepatic diseases. This is particularly true for diseases such as hepatic adenoma (13,14), focal nodular hyperplasia (15–18), hepatoma, and angiosarcoma (19–22), although the relationship between estrogens and focal nodular hyperplasia has been questioned recently (23). Each of these hepatic disorders has been associated, at least circumstantially, with the long-term use of steroidal agents such as estrogens, androgens, or prednisone for any of a variety of clinical indications.
The present study is a report on the identification and quantitation of estrogen receptors in cytosol obtained from normal rat liver and from the Morris hepatoma 7777. The latter is a well-differentiated trabecular carcinoma (24) and was originally induced by feeding N-2-fluorenylphthalmic acid (FPA) to Buffalo strain inbred rats. It is a fast growing “minimal deviation” tumor which grows well in both sexes, although it was maintained primarily in male rats in our laboratory.
Methods
Tissue
Livers from adult male Buffalo rats were used (250 g). The animals were killed by decapitation and the livers were removed, washed, and placed immediately in iced saline solution for later processing. Morris hepatomas 7777 in Buffalo rats were transplanted subcutaneously to Buffalo recipients. The tumors were removed for the preparation of cytosol when the tumor weight was judged to be equal to liver weight (~10 g).
Materials
Estrone (E1), estradiol (E2), estriol (E3), and 2-methoxyestradiol (2-OMe-E2), were purchased from Steraloids, Wilton, N.H. Diethylstilbesterol (DES), testosterone, 5,α-dihydrotestosterone (DHT), progesterone, bovine serum albumin (BSA), standards for gel filtration chromatography, and protamine sulfate were purchased from Sigma Chemical Company, St. Louis, Mo. Norit A and dextran C were obtained from Fisher Scientific Company, Pittsburgh, Pa. Protein A - Sepharose was obtained from Pharmacia Fine Chemicals, Piscataway, N.J.; rabbit antiserum to mouse α-fetoprotein and goat antiserum to rabbit IgG were purchased from Miles Laboratories, Elkhart, Ind. [2,4,6,7,16,17 3H]Estradiol, [3H]E2, 151 Ci/mmol, was obtained from New England Nuclear, Boston, Mass. The radiolabeled material used in these studies was assayed periodically for purity by thin-layer chromatography on silica gel G in ethyl acetate/hexane/ethanol (85:10:5), and was used only if purity as determined by radiolabel migration was 95%.
Preparation of Cytosol
Approximately 10 g of tissue was used for each assay. All procedures were performed at 0°C. The liver was minced and homogenized with a Brinkmann polytron (Brinkmann Instruments, Inc., Westbury, N.Y.) in 2 volumes of TED buffer [0.01 M Tris-HCl, 1.5 mM ethylenediaminetetraacetate (EDTA), 0.01 mM dithiothreitol, pH 7.4]. The resultant homogenate was centrifuged for 60 min at 105,000 g. The supernate was removed carefully to prevent lipid contamination and treated with Norit A and dextran C according to the technique of Chamness (8,25). The final protein concentration in the cytosol samples prepared was 15–25 mg/ml. Immediately before use, the cytosol was diluted to a final protein concentration of 5 mg/ml.
Protamine Sulfate Assay
Receptors were prepared using the protamine sulfate precipitation technique of Chamness et al. (8,25), which precludes contamination due to binding of steroid to the moderate-affinity high-capacity male specific estrogen binder (12). Cytosol (0.2 ml) pretreated with dextran-coated charcoal and protamine sulfate (0.2 ml) solution (1.5 mg/ml in TED buffer) were combined in multiple replicate tubes and mixed briefly. After standing for 5 min at 0°C, the reaction tubes were centrifuged at 800 g for 10 min and the supernate was removed by suction. A 250-μl aliquot of radioactive steroid solution, with or without unlabeled hormone, was then added to each tube. After incubation for 18 h at 0°C, the supernate was removed and the precipitate in each tube was washed with three 2-ml aliquots of cold TED buffer. The precipitate, at the bottom of the reaction tube, was dropped in a scintillation vial with 2 ml of absolute ethanol and mixed for 1 h. After this time the bottoms of the test tubes were removed and 10 ml of Instagel (Packard Instrument Company, Inc., Downers Grove, Ill.) was added.
Binding Studies
Protamine sulfate precipitates of 200-μl aliquots of hepatic or hepatoma cytosol were prepared and incubated with several concentrations of [3H]E2 over a range of 0.15–3 nM in the absence (total binding) and presence (nonspecific binding) of 100-fold excess of unlabeled E2 for 18 h at 0°C. Specific binding was obtained by subtracting nonspecific binding from total binding. Scatchard analysis (26) was performed in the specific binding values. The slope and y-intercept of the apparent linear relationship were determined by use of the unweighted linear regression program of the TE 55 calculator (Texas Instruments, Houston, Tex.). In other studies to assess specificity of binding, a single saturating concentration (1.5 nM) of [3H]E2 was used in the presence and absence of 100-fold excess concentration of various unlabeled hormones, including E2, DES, E1, progesterone, testosterone, and DHT.
Gel Filtration Chromatography
Gel filtration chromatography of cytosolic proteins was carried out on Sephadex G-100 in Tris-EDTA buffer (0.01 M Tris HCl + 1.5 mM EDTA, pH 7.4). Cytosol (4 ml) from hepatoma was incubated with 2 nM [3H]E2 for 2 h before chromatography. A 400-μl aliquot was removed from each 2.5-ml fraction to determine the [3H]E2 content. Cytosol from normal male liver was chromatographed and [3H]E2 binding activity was detected after chromatography by incubating 200 μl of each fraction with 5 nM [3H]E2 for 2 h as described previously (12). The column (2.4 × 45 cm) was previously calibrated with blue dextran, BSA, ovalbumin, trypsinogen, and myoglobin and the molecular weights of the [3H]E2 binding proteins were estimated (27). In other studies on hepatoma, unlabeled cytosol was chromatographed and the [3H]E2 binding activity in each fraction was detected as described previously with unbound steroid being removed by dextran-charcoal treatment (12).
Immunoprecipitation Studies
Protein A-Sepharose was washed and coupled according to the manufacturer’s recommendations to either rabbit antibodies against mouse α-fetoprotein (anti-AFP), or to goat antibodies against rabbit immunoglobulin G, the latter being used as a control preparation. Each preparation was stored as a slurry in TED buffer, 1 volume resin to 7 volumes buffer. Serum from normal and hepatoma-bearing animals, cytosol from normal male rat liver, liver of tumor-bearing hosts, and hepatoma were tested for estrogen binding activity by incubating aliquots (200 μl) of each with 5 nM [3H]E2 for 2 h at 0°C. Unbound steroid was removed with dextran-coated charcoal (12). Serum from host animals and hepatoma cytosol were diluted until binding activity reached 50%–60% of the [3H]E2 added, typically 1:500 for serum and 1:100 for cytosol. Normal rat and host liver cytosol were usually tested without dilution in order to detect low levels of immunoprecipitable estrogen binding. Normal rat serum had no detectable estrogen binding activity. The appropriate dilution of serum or cytosol was then treated with an amount of anti-AFP-Protein A-Sepharose slurry predetermined to result in maximum efficiency of precipitation, usually 0.5 ml, or an equivalent volume of control antiserum–Protein A–Sepharose slurry for 1 h at 0°C. The resin was removed by centrifugation and 200 μl of each supernate was then tested for [3H]E2 binding activity as detailed previously in this section.
Auxiliary Methods
Protein concentration of cytosol was determined by the method of Lowry (28) using BSA as a standard. Radioactivity content of samples was determined in a Packard Tri-carb liquid scintillation spectrometer (Packard Instrument Co., Inc., Downers Grove, Ill.) Multiple results were expressed as mean ± SD and statistical analysis was performed using Student’s nonpaired t-test.
Results
A typical binding curve obtained using the protamine sulfate technique for isolating cytoplasmic estradiol receptors from normal adult male rat liver is shown in Figure 1. Binding is saturable and of limited capacity. Figure 2 demonstrates a plot of the specific binding data using the method of Scatchard for analysis. A single class of binding (receptor) molecules for estradiol with a uniform affinity was found (r = 0.99). In this particular experiment, the binding capacity was determined to be 35.8 fmol E2/mg protein and the Kd was determined to be 4.42 × 10−10 M. Next, the level of estrogen receptors was assessed in several normal livers and in hepatoma tissue as described in Figures 1 and 2. Figure 3 shows the specific binding of normal male rat liver (six samples) and that of Morris hepatoma 7777 (six samples). In four of the hepatomas the estrogen receptors were not found, that is, no specific binding was detectable. Two other hepatomas contained small amounts of estrogen receptor (0.26 and 2.2 fmol/mg protein), much less than that detected in the normal liver. Other samples of hepatoma cytosol have been tested for retention on heparin-Sepharose, an affinity resin for steroid receptors; no estrogen binding activity was retained by this resin.
Figure 1.
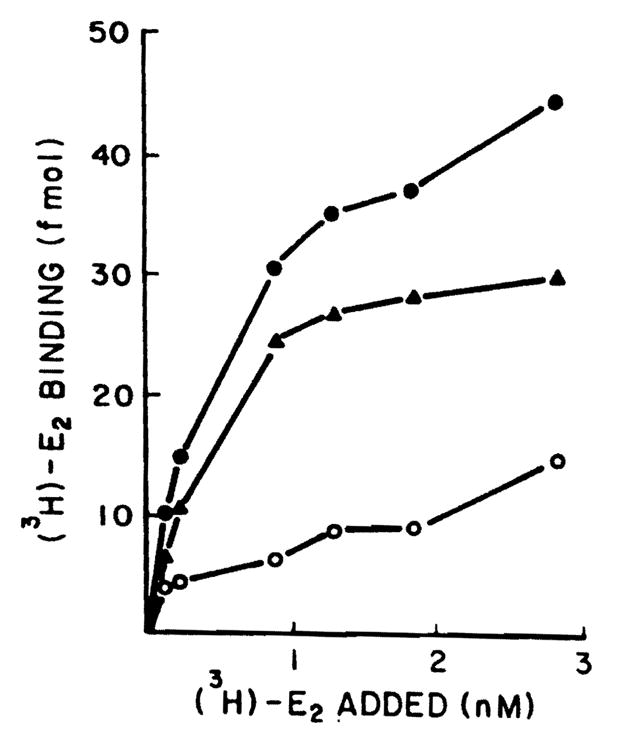
Specific binding of the [3H]E2 by cytosol of the normal male rat liver. Aliquots (200 μm) of whole cytosol (5 mg/ml) precipitated with protamine sulfate were incubated with six different concentrations of [3H]E2 (0.15–3 nM) for 18 h at 0°C in the presence and absence of 100-fold unlabeled E2. Specific binding was calculated by subtracting nonspecific binding from total binding. Each point is the average of triplicate determinations. Closed circles represent total binding, open circles nonspecific binding, triangles specific binding.
Figure 2.
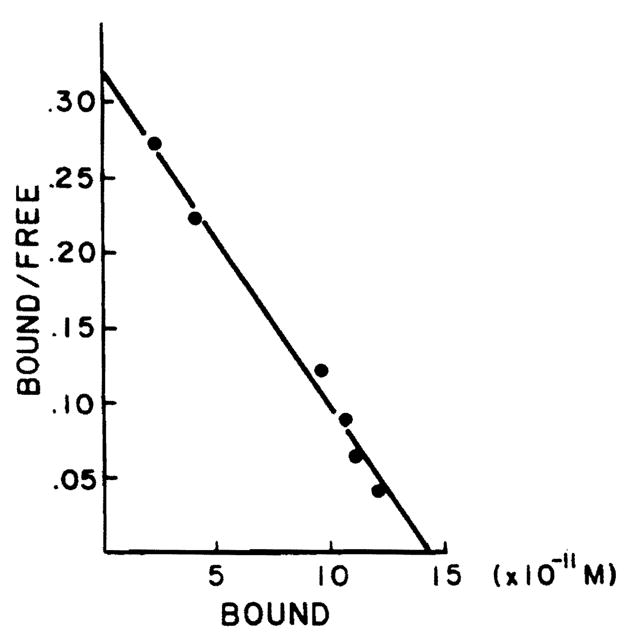
Scatchard plot analysis of specific [3H]E2 binding in protamine sulfate precipitates of whole rat male liver cytosol. Aliquots (200 μl) of whole cytosol were precipitated with protamine sulfate and were incubated with six different concentrations of [3H]E2 (0.15–3 nM) for 18 h at 0°C in the presence and absence of 100-fold unlabeled E2.
Figure 3.
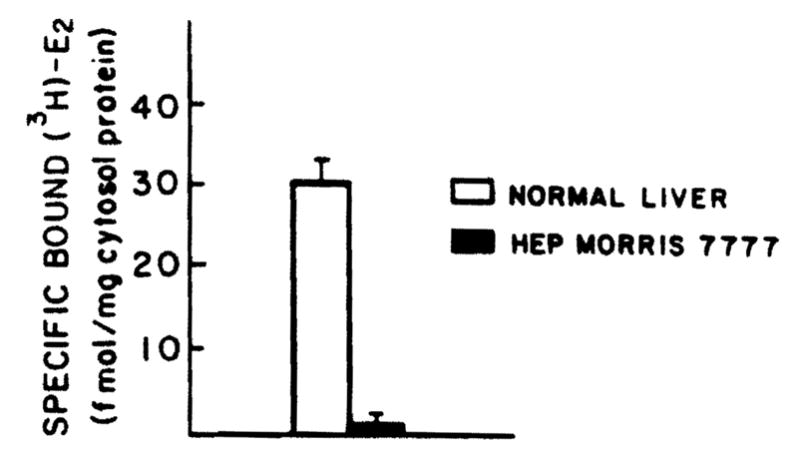
Specific binding of the [3H]E2 by cytosol from adult male rats and Morris hepatoma 7777. Aliquots (200 μl) of whole cytosol (5 mg/ml) were precipitated with protamine sulfate and were incubated with 1.5 mM of [3H]E2 in the presence or absence of 100-fold unlabeled E2. Specific binding was calculated by subtracting nonspecific binding from total binding and was expressed as femtomoles per milligram of cytosol protein. p < 0.001.
To establish that the binding of the labeled estrogen in these studies was specific, the ability of various unlabeled steroids to compete with labeled estradiol was determined. The data shown in Figure 4 demonstrate that the binding was specific for estrogens. Thus. [3H]E2 binding was not inhibited by testosterone, DHT, and progesterone.
Figure 4.
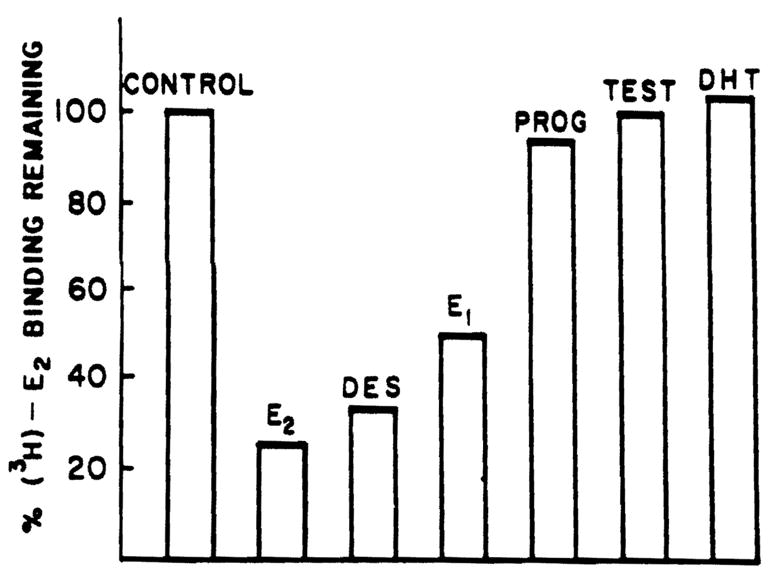
Specificity of binding to cytosolic proteins in male rat liver cytosol. Aliquots (200 μl) of whole male rat liver cytosol were precipitated with protamine sulfate and were incubated with 1.5 nM [3H]E2 in the presence and absence of 100-fold excess of competing substance for 18 h at 0°C. Each bar represents triplicate determinations.
While protamine sulfate precipitation of hepatoma cytosol resulted in the detection of little or no estrogen receptor, estrogen binding studies in whole hepatoma cytosol revealed the presence of a high-capacity estrogen binder. Binding studies show that this protein has a Kd for E2 of 5 × 10−8 M, indicating moderate affinity, and has a very high E2 binding capacity, 20 pmol/mg cytosol protein (data not shown).
When whole cytosol from normal liver and hepatoma were tested for estrogen binder content using gel filtration chromatography, the results were strikingly different for these two tissues. As shown in Figure 5, normal rat liver has two [3H]E2 binding species. The first, eluting in the void volume of the column, has been characterized extensively as the hepatic estrogen receptor (12). The second, eluting from the column at a position consistent with a molecular weight of 25,000 is the hepatic male specific estrogen binder (MEB) described previously (12). Protamine sulfate precipitates the receptor but not the MEB (12). In contrast, the hepatoma contains neither the receptor nor the MEB, but instead contains a unique estrogen binder which elutes as a species of ~70,000 molecular weight. Further analysis of this material pooled from fractions 34–36 after Sephadex G-100 chromatography reveals that unlabeled DES does not compete for [3H]E2 binding to this protein (Figure 6). Diethylstilbesterol, however, is very effective in competing for [3H]E2 binding to the receptor of normal liver as shown in Figure 4, suggesting that the protein which is present in hepatoma cytosol is not an altered form of the receptor. In addition, the specificity of the hepatoma estrogen binder differs from the MEB in that neither E3 nor 2-OMe-E2 competes for [3H]E2 binding to the hepatoma protein; both of these substances are avid competitors of [3H]E2 binding to MEB (12. manuscript in preparation). Furthermore, while protamine sulfate effectively precipitates the receptor of normal liver, it does not precipitate this hepatoma estrogen binder (Figure 6).
Figure 5.
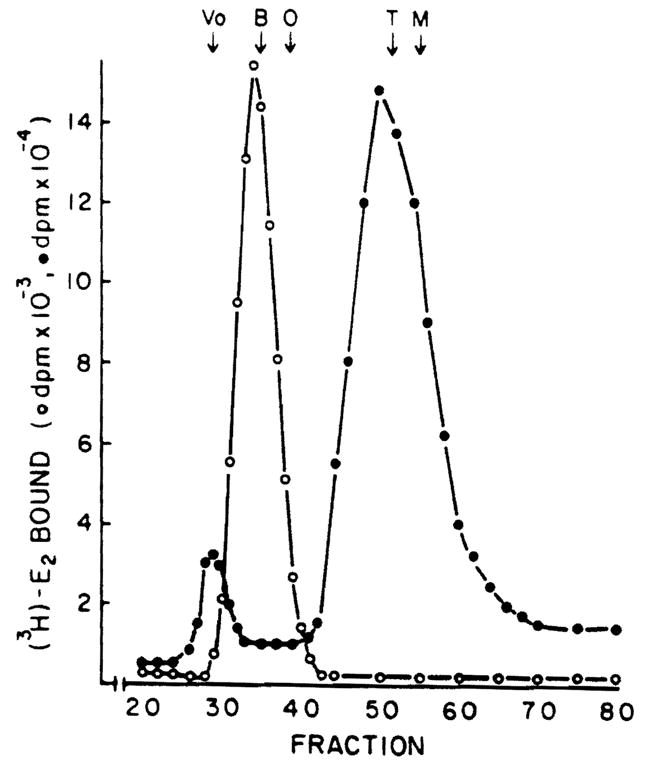
Gel filtration chromatography of estrogen binding proteins from male rat liver and Morris hepatoma 7777 cytosol. Cytosol (4 ml) from normal liver (open circles) was chromatographed on Sephadex G-100 and estrogen binders were detected as indicated in Methods. Hepatoma (open circles) cytosol was incubated for 2 h with 2 nM [3H]E2 before chromatography on Sephadex G-100. A 400-μl aliquot of each fraction was assayed for [3H]E2 content. Markers used to calibrate the column included blue dextran (Vo); bovine serum albumin (B), 68.000; ovalbumin (O), 43,000; trypsinogen (T), 24,000; myoglobin (M), 17,000.
Figure 6.
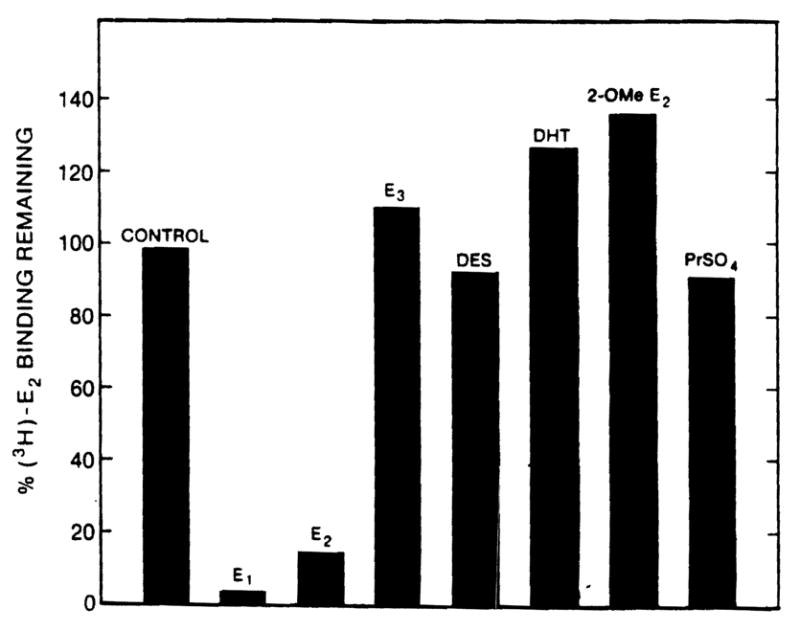
Specificity of binding to hepatoma estrogen binder. Fractions 33–35 were pooled from an experiment identical to that illustrated in Figure 5 except that the hepatoma cytosol was not labeled with [3H]E2 before chromatography; instead, each fraction was tested for [3H]E2 binding activity as indicated in Methods. The pooled fractions were tested for specificity by incubating with 5 nM [3H]E2 in the absence or presence of a 100-fold excess of the potentially competing substance for 16 h at 0°C. In one experiment, the pooled fractions were labeled with [3H]E2 as above, except that after incubation and dextran-coated charcoal treatment, the sample was treated with protamine sulfate and the supernatant estrogen binding activity was determined.
A protein known to bind estrogen with similar affinity and specificity, and of a molecular size similar to the estrogen binder in hepatoma is α-fetoprotein (AFP) (29). Therefore, we treated cytosol from normal and host liver as well as from hepatoma with antiserum to AFP; the results are shown in Table 1. Treatment of hepatoma cytosol with anti-AFP results in removal of most of the estrogen binding activity (82.4%), whereas control antiserum had no effect. Undiluted host liver cytosol contains some immunoprecipitable E2 binding activity, probably as a result of serum contamination, as the serum of the host animal contains large quantities of AFP activity. Normal rat liver cytosol, undiluted, or at a 1: 10 dilution (not shown), demonstrated no loss of [3H]E2 binding activity in the presence of either antiserum.
Table 1.
Immunoprecipitation of [3H]E2 Binding Activity in Hepatoma, Serum, and Normal Liver
| Preparation | Dilution | [3H]E2 binding activity remaining |
|
|---|---|---|---|
| Control antiseruma (%) | AFP-antiserum (%) | ||
| Hepatoma cytosol | 1:100 | 100 | 17.6 |
| Host liver cytosol | Undiluted | 100 | 73.1 |
| Host serum | 1:500 | 100 | 23.9 |
| Normal liver cytosol | Undiluted | 100 | 98.6 |
AFP = α-fetoprotein.
Values for control serum-treated samples were set at 100%; these values were within ± 10% of values of [3H]E2 binding in samples that were not treated with any Protein A-antibody resin, but were instead diluted with an appropriate volume of buffer.
Discussion
The tumor-inducing potential of hormones in endocrine-related organs of experimental animals has been well demonstrated. Ever since Eisenfeld et al. (5,11) initially reported the presence of specific estradiol binding sites in the hepatic cytosol of adult female and male rats, and the subsequent demonstration of the presence of a molecule described as an androgen receptor in the same tissue by Milin et al. (30), many research teams (31–34) have attempted to determine the relationship between hepatic steroid binding and the development of benign and malignant hepatic neoplasms.
In 1973, Baum et al. (13) suggested a relationship between benign hepatic tumors and focal nodular hyperplasia and estrogen use in women who had a history of prior oral contraceptive use. In contrast to the usual relatively benign nature of contraceptive-(estrogenic) induced tumors, liver tumors associated with the use of anabolic steroids are usually malignant, especially when the androgen content of the hormonal preparation is high (35–37).
At the present time, anabolic, androgenic, and estrogenic hormones are used widely for a variety of clinical indications. Because of this widespread use, numerous experiments have been performed to define the role of steroids, especially estrogens, in the pathogenesis of liver cell tumors. As a result of such studies, some authors have suggested that estrogens are carcinogenic (31,33,34). For example, in animals given various preparations of estrogens and progestational agents, the liver tumor occurrence rate can be varied from 0% to 60% depending on the dose, agents given, and the time of exposure (34).
Other authors have suggested that estrogens promote the carcinogenic effects of various procarcinogens but that they alone are not intrinsically carcinogenic (38–41). With such a hypothesis in mind, Wanless et al. (42) recently studied the diethylnitrosamine-pretreated rat model. In these experiments it was shown that estrogens promote the development of hepatic neoplasm by a mechanism which involves increasing the mitogenic activity of the hepatocyte nucleus. It should be noted, however, that the dosage of estrogen used in these experiments was 200-fold greater than that used clinically.
On the other hand, the biological importance of the estrogens in the process of malignant transformation of hepatic tumors has been questioned by Mishkin et al. (43). These authors show that administration of estrogen and tamoxifen in combination results in a reversal of acetylaminofluorine-induced hepatic hypertrophy, nodular proliferation, and malignant transformation. These chemically induced nodules before estrogen treatment displayed decreased estrogen receptor levels as compared with normal liver; however, the effects of the subsequent estrogen treatment on receptor levels were not elucidated. It is generally conceded that hormone receptors are necessary for the maintenance of hormone dependency. At this time, however we are uncertain as to which factors control receptor levels, and further, what role these receptors play in the disease process.
Therefore, it may be premature to explain the relationship, if one exists, between the estrogen binding capacity of normal liver and experimental tumor or experimental estrogen-induced tumors. It is possible that the estrogen receptor is essential for participation in the early events of tumor formation, but that in later stages the estrogen, through its receptor, no longer exerts an influence on the cell. The present study clearly establishes that the Morris hepatic tumor does not have an increased estrogen receptor capacity or increased affinity for estrogens compared with normal rat liver. In contrast, the data clearly document reduced (p < 0.001) or no receptor binding activity by the tumor as compared with normal tissues. The lack of receptors in the transplantable Morris hepatoma 7777 may represent an endpoint of dedifferentiation in this tissue. In fact, estrogen receptors have been detected in human adenoma, but at a level significantly lower than in normal tissue from the same liver (44). Further studies are necessary to confirm such a temporal relationship.
The estrogen binder that is present in hepatoma cytosol but not in protamine sulfate precipitates or normal liver is interesting. This estrogen binder has properties that suggested that it may be AFP. Its molecular weight is comparable to AFP (45,46) and its specificity is such that DES does not compete for E2 binding, unlike the classic estrogen receptor (47). We have confirmed its identity by its selective immunoprecipitation by antiserum to AFP. These studies, however, also point to a necessary caution; estrogen binding in unfractionated liver cytosol, particularly in abnormal tissue, does not necessarily indicate the presence of an estrogen receptor. It is essential to establish the presence of receptor by criteria other than simply its ability to bind estrogen.
Acknowledgments
This study was supported by grants from the Veterans Administration, by project grant AM 29961 and AM 30001 from the National Institutes of Health, and by grant 82/0031096 from Consiglio Nazionale Delle Ricerche, Italy.
Abbreviations used in this paper
- AFP
α-fetoprotein
- BSA
bovine serum albumin
- DHT
5,α-dihydrotestosterone
- DES
diethylstilbesterol
- E1
estrone
- E2
estradiol
- E3
estriol
- FPA
N-2-fluorenylphthalmic acid
- 2-OMe-E2
2-methoxyestradiol
References
- 1.Corvol PL, Chrambach A, Rodbard D, et al. Physical properties and binding capacity of testosterone-binding globulin in human plasma determined by polyacrylamide electrophoresis. J Biol Chem. 1971;246:3435–43. [PubMed] [Google Scholar]
- 2.Laragh JH, Bauer L, Brunner HR, et al. Renin angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med. 1972;52:633–52. doi: 10.1016/0002-9343(72)90054-x. [DOI] [PubMed] [Google Scholar]
- 3.Menard J, Corvol P, Foliot A, et al. Effects of estrogens on renin substrate and uterine weights in rats. Endocrinology. 1973;93:747–51. doi: 10.1210/endo-93-3-747. [DOI] [PubMed] [Google Scholar]
- 4.Song CS, Rifkind AB, Gillete PN, et al. The effect of estrogens, progestins and pregnancy on hepatic function. Am J Obstet Gynecol. 1969;105:813–47. [PubMed] [Google Scholar]
- 5.Eisenfeld AJ, Aten RF, Weinberger M, et al. Estrogen receptor in the mammalian liver. Science. 1976;191:862–5. doi: 10.1126/science.175442. [DOI] [PubMed] [Google Scholar]
- 6.Eisenfeld AJ, Aten RF, Haselbacher GK, et al. Specific macromolecular binding of estradiol in the mammalian liver supernate. Biochem Pharmacol. 1977;26:919–24. doi: 10.1016/0006-2952(77)90466-x. [DOI] [PubMed] [Google Scholar]
- 7.Viladiu P, Delgado C, Pensky J, et al. Estrogen binding protein of rat liver. Endocr Res Commun. 1975;2:273–80. doi: 10.3109/07435807509053854. [DOI] [PubMed] [Google Scholar]
- 8.Chamness GC, Costlow ME, McGuire WL. Estrogen receptor in rat liver and its dependence on prolactin. Steroids. 1975;26:363–71. doi: 10.1016/0039-128x(75)90081-1. [DOI] [PubMed] [Google Scholar]
- 9.Powell-Jones W, Davies P, Griffiths K. Specific binding of (3H)-estradiol by cytoplasmic protein components of female liver. J Endocrinol. 1976;69:167–8. doi: 10.1677/joe.0.0690167. [DOI] [PubMed] [Google Scholar]
- 10.Beers PC, Rosner W. The binding of estrogens in the liver of the rat: demonstration and endocrine influences. J Steroid Biochem. 1977;8:251–8. doi: 10.1016/0022-4731(77)90017-6. [DOI] [PubMed] [Google Scholar]
- 11.Aten RF, Dickson RB, Eisenfeld AJ. Estrogen receptor in adult male rat liver. Endocrinology. 1978;103:1629–35. doi: 10.1210/endo-103-5-1629. [DOI] [PubMed] [Google Scholar]
- 12.Eagon PK, Fisher SE, Imhoff AF, et al. Estrogen binding proteins of male rat liver: influences of hormonal changes. Arch Biochem Biophys. 1980;201:486–99. doi: 10.1016/0003-9861(80)90537-8. [DOI] [PubMed] [Google Scholar]
- 13.Baum JK, Holtz F, Bookstein JJ, et al. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;ii:926–9. doi: 10.1016/s0140-6736(73)92594-4. [DOI] [PubMed] [Google Scholar]
- 14.Edmondson HA, Henderson B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470–2. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 15.Fechner RE. Benign hepatic lesions and orally administered contraceptives. A report of seven cases and a critical analysis of the literature. Hum Pathol. 1977;8:255–68. doi: 10.1016/s0046-8177(77)80022-1. [DOI] [PubMed] [Google Scholar]
- 16.Ishak KG. Primary hepatic tumors in childhood. In: Popper H, Schaffner F, editors. Progress in liver diseases. New York: Grune & Stratton; 1976. pp. 636–67. [PubMed] [Google Scholar]
- 17.Ishak KG, Rabil L. Benign tumors of the liver. Med Clin North Am. 1975;59:995–1013. doi: 10.1016/s0025-7125(16)31998-8. [DOI] [PubMed] [Google Scholar]
- 18.Knowles DM, Wolff M. Focal nodular hyperplasia of the liver. A clinicopathologic study and review of the literature. Hum Pathol. 1976;7:533–45. doi: 10.1016/s0046-8177(76)80101-3. [DOI] [PubMed] [Google Scholar]
- 19.Christopherson WM, Mays ET, Barrows GH. Liver tumors in women on contraceptive steroids. Obstet Gynecol. 1975;46:221–3. [PubMed] [Google Scholar]
- 20.Christopherson WM, Mays ET, Barrows GH. A clinicopathologic study of steroid related tumors. Am J Surg Pathol. 1977;1:31–41. doi: 10.1097/00000478-197701010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Neuberger J, Nunnerley HB, Davis M, et al. Oral contraceptive-associated liver tumors: occurrence of malignancy and difficulties in diagnosis. Lancet. 1980;i:273–6. doi: 10.1016/s0140-6736(80)90776-x. [DOI] [PubMed] [Google Scholar]
- 22.Mays ET, Christopherson WM, Mahr MM, et al. Hepatic changes in young women ingesting contraceptive steroids: hepatic hemorrhage and primary hepatic tumors. JAMA. 1976;235:730–2. [PubMed] [Google Scholar]
- 23.Kerlin P, Davis GL, McGill DB, et al. Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic, and radiologic features. Gastroenterology. 1983;84:994–1002. [PubMed] [Google Scholar]
- 24.Morris HP, Wagner BP. Induction and transplantation of rat hepatomas with different growth rate (including “minimum deviation” hepatomas) In: Busch H, editor. Methods in cancer research. New York: Academic; 1968. pp. 125–52. [Google Scholar]
- 25.Chamness GC, Huff K, McGuire WL. Protamine-precipitated estrogen receptor: a solid-phase ligand exchange assay. Steroids. 1975;25:627–35. doi: 10.1016/0039-128x(75)90017-3. [DOI] [PubMed] [Google Scholar]
- 26.Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–72. [Google Scholar]
- 27.Erdos T, Fries J. The subunit-structure of the uterine “oestradio receptor. Biochem Biophys Res Commun. 1974;58:932–9. doi: 10.1016/s0006-291x(74)80233-0. [DOI] [PubMed] [Google Scholar]
- 28.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 29.Baker ME, Frecker DG, Fanestil DD. Inhibition of estrogen binding to rat alpha-fetoprotein by tryptophan p-nitrophenyl esters. J Steroid Biochem. 1982;16:503–7. doi: 10.1016/0022-4731(82)90070-x. [DOI] [PubMed] [Google Scholar]
- 30.Milin B, Roy AK. Androgen “receptor” in rat liver: cytosol “receptor” deficiency in pseudohermaphrodite male rats. Nature New Biol. 1973;242:248–50. doi: 10.1038/newbio242248a0. [DOI] [PubMed] [Google Scholar]
- 31.Committee on safety of medicines. Carcinogenicity tests of oral contraceptives. London: HM Stationery Office; 1972. [Google Scholar]
- 32.Hertz R. A review of the evidence linking estrogens and cancer in animals. Pediatrics. 1978;62:1138–42. [PubMed] [Google Scholar]
- 33.Reznik-Schuller H. Carcinogenic effects of diethylstilbestrol in male Syrian golden hamsters and European hamsters. J Natl Cancer Inst. 1979;62:1083–8. [PubMed] [Google Scholar]
- 34.Schardein JL, Kaump DH, Woosley ET. Long term toxicologic and tumorogenesis studies on an oral contraceptive agent in albino rats. Toxicol Appl Pharmacol. 1970;16:10–23. doi: 10.1016/0041-008x(70)90157-2. [DOI] [PubMed] [Google Scholar]
- 35.Ishak KG. Hepatic lesions caused by anabolic and contraceptive steroids. Sem Liver Dis. 1981;1:116–28. doi: 10.1055/s-2008-1040724. [DOI] [PubMed] [Google Scholar]
- 36.Johnson FL. Androgenic-anabolic steroids and hepatocellular carcinoma. In: Okuda K, Peters RL, editors. Hepatocellular carcinoma. New York: John Wiley & Sons; 1976. pp. 95–103. [Google Scholar]
- 37.Christopherson WM, Mays ET, Barros CH. Liver tumors in young women: a study of 201 cases in the Louisville registry. In: Fenoglio CM, Wolff M, editors. Progress in surgical pathology. II. New York: Masson USA; 1980. pp. 187–205. [Google Scholar]
- 38.Reuber MD. Influence of hormones on N-2-fluorenyldiacetamide-induced hyperplastic hepatic nodules in rats. Natl Cancer Inst. 1969;43:445–52. [PubMed] [Google Scholar]
- 39.Sumi C, Yokoro K, Kajitani T, et al. Synergism of diethylstilbestrol and other carcinogens in concurrent development of hepatic, mammary, and pituitary tumors in castrated male rats. J Natl Cancer Inst Monogr. 1980;65:169–75. [PubMed] [Google Scholar]
- 40.Taper HS. The effect of estradiol-17-phenylpropionate and estradiol benzoate on N-nitrosomorpholine-induced liver carcinogens in ovariectomized female rat. Cancer. 1978;42:462–7. doi: 10.1002/1097-0142(197808)42:2<462::aid-cncr2820420213>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 41.Yager JD, Yager R. Oral contraceptive steroids as promoter of hepatocarcinogenesis in female Sprague–Dawley rats. Cancer Res. 1980;40:3680–5. [PubMed] [Google Scholar]
- 42.Wanless IR, Medline A. Role of estrogens as promoters of hepatic neoplasia. Lab Invest. 1982;46:313–20. [PubMed] [Google Scholar]
- 43.Mishkin SY, Farber E, Kun HR, et al. Evidence for the hormone dependency of hepatic hyperplastic modules: inhibition of malignant transformation after exogenous 17-α-estradiol and Tamoxifen. Hepatology. 1983;3:308–16. doi: 10.1002/hep.1840030306. [DOI] [PubMed] [Google Scholar]
- 44.Porter LE, Elm MS, Van Thiel DH, Eagon PK. Estrogen receptor in human liver disease (abstr) Clin Res. 1983;31:287A. doi: 10.1016/0016-5085(87)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Aussel C, Uriell J, Mercier-Bodard C. Rat alpha-fetoprotein isolation characterization and estrogen-binding properties. Biochimie. 1973;55:1431–7. doi: 10.1016/s0300-9084(74)80550-x. [DOI] [PubMed] [Google Scholar]
- 46.Sell S. Alpha-fetoprotein. In: Sell S, editor. Cancer markers, diagnostic and developmental significance. Clifton, N.J: The Humana Press; 1980. pp. 249–93. [Google Scholar]
- 47.Chen TL, Feldman D. Distinction between alpha-fetoprotein and intracellular estrogen receptors: evidence against the presence of estradiol receptors in rat bone. Endocrinology. 1978;102:236–44. doi: 10.1210/endo-102-1-236. [DOI] [PubMed] [Google Scholar]


