Figure 3. Model of the molecular steps mediated synaptic vesicle exocytosis (modified from [38]).
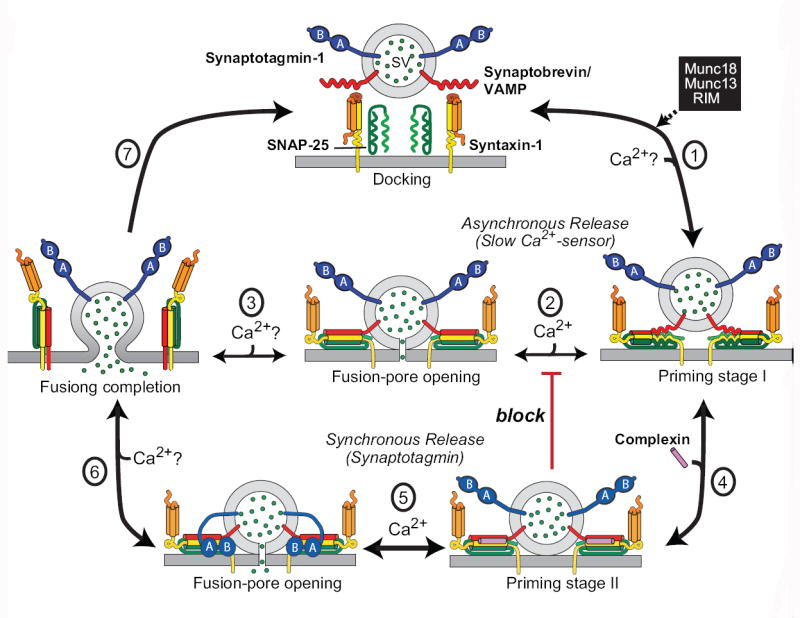
Synaptic vesicles are docked at the active zone of a presynaptic terminal with unassembled SNARE complexes (top)m and are then primed for release by partial SNARE-complex assembly that is catalyzed by Munc18, Munc13, and RIM (step 1). At least in inhibitory synapses, this priming process might be further modulated by ELKS2. The primed vesicles form the substrate for two main pathways of Ca2+-triggered neurotransmitter release: asynchronous release (steps 2 and 3), in which full assembly of SNARE complexes leads to fusion-pore opening followed by complete fusion (step 3); and synchronous release (steps 4,5 and 6), in which “superpriming” by binding of complexins to assembled SNARE complexes (step 4) activates and freezes SNARE complexes in a metastable state (referred to as priming stage II). This stage is then substrate for fast Ca2+-triggering of release when Ca2+-binding to Syt1 induces its binding to phospholipids and to SNARE complexes, with the latter reaction displacing complexin and resulting in fusion-pore opening (step 5) and full fusion (step 6). Both the synchronous and the asynchronous release pathway can mediate spontaneous ‘mini’ release, depending on the local Ca2+-microdomain. Synaptotagmin and complexin clamp (block, in red) the unidentified slow Ca2+-sensor which mediates the asynchronous release; this clamping is relieved when Ca2+ binds to Syt1, allowing competition between Syt1 and the asynchronous Ca2+-sensor during high-frequency stimulation [12,44].
