Fig. 1.
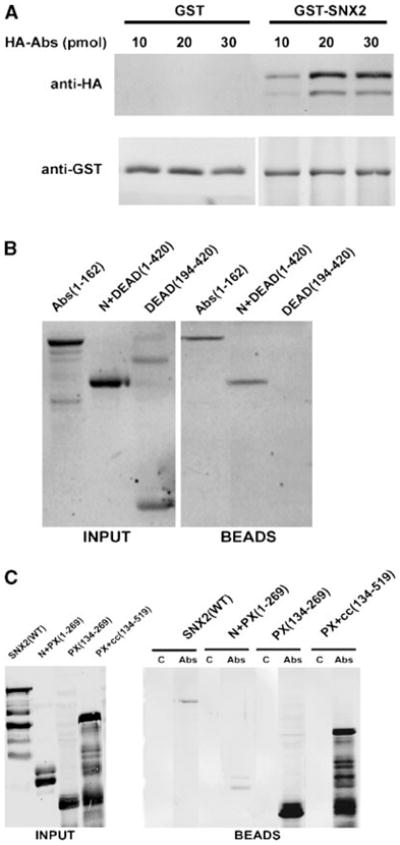
In vitro pulldown of Abstrakt (Abs) and sorting nexin-2 (SNX2). A: Increasing amounts of recombinant hemagglutinin (HA)-Abs was added to 5 pmol of GST-SNX2 or GST pre-bound to glutathione-agarose beads. Proteins bound to the beads were analyzed with anti-HA (top part) and anti-GST (bottom part) antibodies. B: Various HA-Abs deletion fragments (20 pmol each) were added to GST-SNX2 bound to glutathione-Sepharose beads. Numbers in parentheses indicate the inclusive range of amino acids in each Abs fragment. HA-Abs in the input (left part) and bound to the beads (right part) were analyzed with anti-HA. Fraction of the Abs DEAD(194-420) fragment often appear as a SDS-resistant dimer. C: Various FLAG-SNX2 fragments (labeled input, left part) were added to control Sepharose 4B beads (lanes labeled C, right part) or to equal volume of Abs immobilized on Sepharose 4B (lanes labeled Abs, right part). FLAG-SNX2 associated with the beads was detected with anti-FLAG antibodies.
