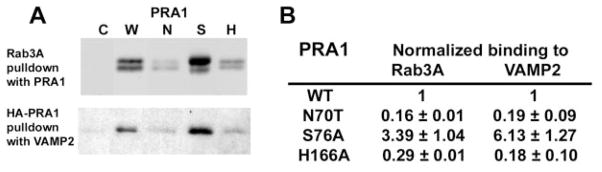
Fig. 7. In vitro binding of Rab3A and VAMP2 to PRA1.
A, representative Western immunoblot using anti-Rab3A (upper panel) and anti-HA (lower panel). Immobilized PRA1s was used for Rab3A pulldown, and glutathione-agarose was used to recover GST-VAMP2. Control beads (C), wild type PRA1 (W), N70T (N), S76A (S), or H166A (H). B, binding of Rab3A and VAMP2 to the mutant PRA1s normalized to that of the wild type PRA1. Values represent mean and S.E. (n = 3 with each performed in triplicate).