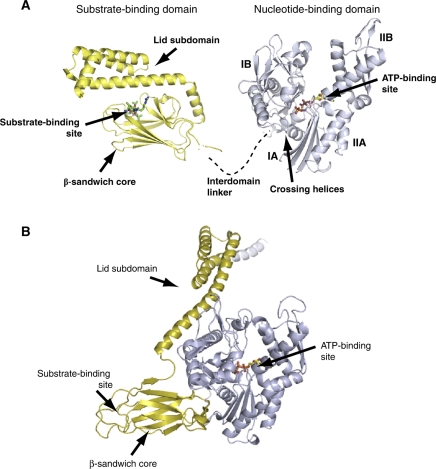
Figure 1.
A model for Hsp70 interdomain allostery. (A) In the ADP-bound state, nucleotide-binding and substrate-binding domains tumble independently of one another, the hydrophobic interdomain linker is relatively exposed, the β-sandwich sub-domain is relatively ordered, the lid sub-domain is closed, and substrate binds with high affinity (PDB codes 1DKG and 1DKZ). (B) ATP binding is accompanied by conformational changes within the nucleotide-binding domain, domain docking with participation of the interdomain linker, opening of the lid sub-domain, reduction in order at the substrate-binding site within the β-sandwich, and loss of substrate-binding affinity.