Figure 2.
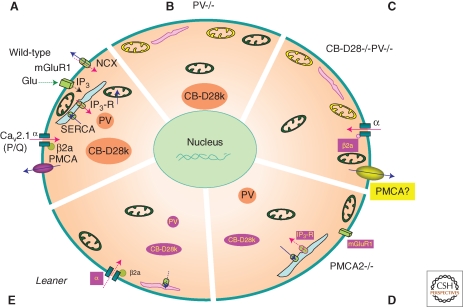
Homeostatic/adaptive changes in the soma of Purkinje cells (PC) caused by malfunctioning or elimination of Ca2+-signaling toolkit component(s); regulation by the Ca2+ homeostasome. (A) A detailed situation is depicted for wild-type mice. Increases in [Ca2+]i (red arrows) result from influx via CaV2.1 (P/Q) channels or release from internal stores (light blue) via the IP3 receptor. IP3 is generated by the activation of metabotropic glutamate receptors (mGluR). Ca2+ removal systems (blue arrows) include PMCA and NCX in the plasma membrane, SERCA pumps, and mitochondria (green). Identified Ca2+-signaling toolkit components including organelles, which are up- or down-regulated, are marked in yellow and magenta, respectively. (B) In PV−/−, PC subplasmalemmal mitochondria are increased, while ER volume directly underneath the plasma membrane is decreased. (C) In addition to the changes observed in PV−/−, in PC lacking both, PV and CB-D28k the auxiliary Cavβ2a subunit of CaV2.1 (P/Q), is decreased, leading to increased voltage-dependent inactivation of P-type currents. Model studies indicate an up-regulation of Ca2+ extrusion systems, possibly PMCA. (D) In PMCA2−/− PC, expression of mGluR1 and of IP3 receptor type 1 (IP3R1), responsible for the Ca2+ release from ER stores, is decreased. Also, the cytosolic Ca2+ buffering capacity mediated by CB-D28k is decreased. (E) In leaner mice PC that are characterized by strongly attenuated Cav2.1 Ca2+ channel function, the rapid Ca2+ buffering/sequestering capacity is reduced: PV and CB-D28k are down-regulated and (subplasmalemmal) ER is decreased/impaired, leading to reduced Ca2+ uptake.