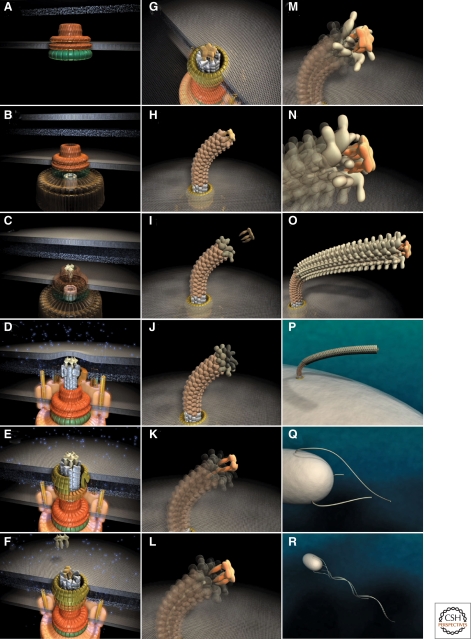
Figure 3.
Steps in assembly of the bacterial flagellum. The self-assembly process of the bacterial flagellum starts from the top left (A) and proceeds to the bottom right (R). Assembly starts with the formation of the MS-ring in the cytoplasmic membrane (A). Afterward, the cytoplasmic C-ring is attached to the MS-ring and the flagellar-specific type III secretion apparatus assembles within a central pore of the MS-ring (B). Flagellar secretion substrates are now secreted specifically via the T3S apparatus. Flagellar proteins that reach the distal end of the growing structure, self-assemble onto the existing structure with the help of distal cap proteins (shown as pentamers at the tips of the axial structures) (C–O). First, the rod acting as a driveshaft is assembled beneath the rod-cap, which is also a muramidase to allow penetration through the peptidoglycan layer (D). In addition, motor force generators that couple proton flow to torque generation assemble in the cytoplasmic membrane and interact with C-ring components (D). Rod assembly ends with the formation of the PL-ring bushing, outer membrane penetration, and the replacement of the rod scaffold with the hook scaffold (E–G). Afterward, hook subunits are secreted and the hook grows to a final length of 55 nm (Hirano et al. 1994), triggering a secretion specificity switch from rod-hook-type substrates to late-secretion substrates (H). Upon completion of the hook-basal-body complex, hook-filament junction proteins (I–J), the filament cap (K–L), and filament subunits are secreted, and the filament grows to a maximal length of about 10–15 µM (M–R). (Reprinted, with permission, from Minamino et al. 2008a [Royal Society of Chemistry].)