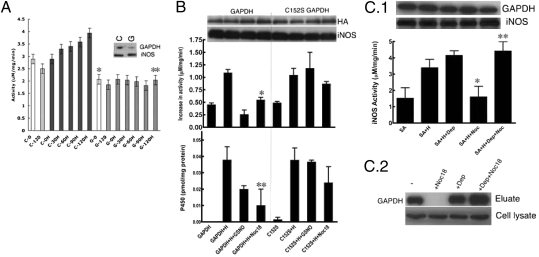
Fig. 3.
Expression level and S-nitrosylation of GAPDH controls cellular heme insertion. (A) GAPDH-silenced SA-treated RAW264.7 cells were unable to insert heme (H) into apo-iNOS as measured by the gain in NO synthesis activity achieved over a 2-h (0–120 min) incubation of cells with heme. GAPDH (G) silenced vs. control (C) silenced (n = 3, mean ± SEM; *P = 0.006 vs. C0; **P = 0.008 vs. C120H by two-tailed Student's t test at equal variance). The inset shows the cells contained a reduced amount of GAPDH protein after silencing GAPDH (G) compared with control siRNA (C). Levels of iNOS protein expression were unaffected. (B) Overexpression of C152S GAPDH but not GAPDH makes cellular heme (H) insertion into apo-iNOS resistant to NO donor (400 μM GSNO or 250 μM Noc18) as measured by the gain in NO synthesis activity or P450 spectral measure of iNOS heme content (n = 3, mean ± SEM; *P = 0.024 vs. GAPDH+H; **P = 0.008 vs. GAPDH+H by two-tailed Student's t test at equal variance). Cell levels of GAPDH or iNOS protein were equivalent across the various treatments. (C.1) Addition of 5 nM deprenyl to the cell culture made heme insertion into apo-iNOS resistant to the inhibition by the NO donor Noc18 (n = 3, mean ± SEM; *P = 0.0101 vs. SA+H; and **P = 0.0222 vs. SA+Noc18 by two-tailed paired Student's t test). Protein levels were unaffected by the treatments as judged by Western blots. (C.2) The presence of 5 nM deprenyl in cultures of RAW 264.7 cells made the heme-binding property of cellular GAPDH resistant to Noc18, but did not affect GAPDH expression, as shown by hemin-agarose pull-downs and Western blotting for GAPDH.