Figure 1.
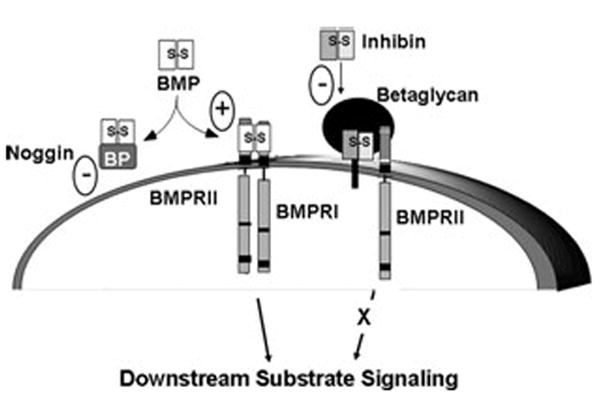
Inhibin antagonism of receptor serine kinase signaling via β-glycan (transforming growth factor [TGF]-β RIII). Bone morphogenetic protein (BMP) signals through the heteromeric type II and type I receptor serine kinases. BMP binds BMPRII, which in turn recruits and phosphorylates BMPRI to stimulate cytoplasmic downstream substrate signaling, primarily through phosphorylation of SMADs 1, 5, and 8. This action can be blocked by soluble BMP antagonists, such as noggin, and enhanced by BMP–β-glycan interaction. Inhibins can also antagonize receptor serine kinase signaling. Inhibin binds with high affinity to β-glycan, allowing for a complex formation with BMPRII. Inhibin–β-glycan–BMPRII interactions sequester BMPRII, preventing its subsequent recruitment of BMPRI and downstream substrate signaling, such as SMAD phosphorylation.
