Figure 3. Pro-cath-D binds to residues (349-394) of LRP1β in vitro.
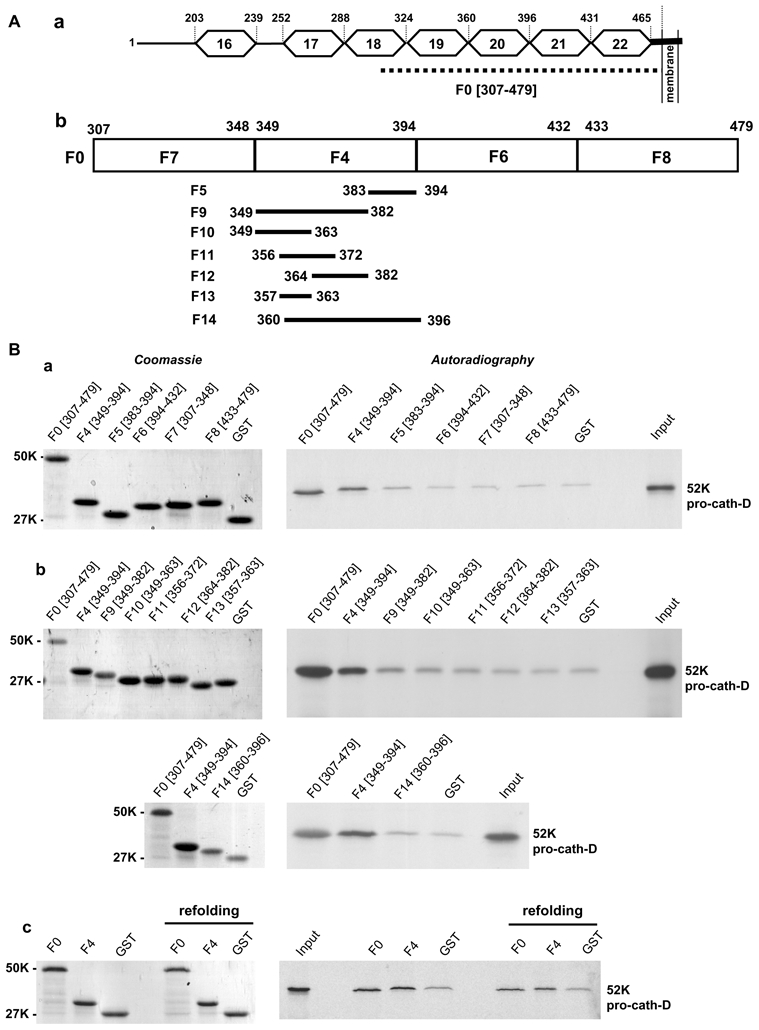
(A) Schematic representation of the LRP1β extracellular domain and GST-LRP1 β fragments. A schematic representation of EGF-like repeats 16 to 22 located in the full-length extracellular domain of LRP1β is shown in panel a. The F0 (307-479) fragment, which interacts with cath-D, is indicated by a dotted line. GST-LRP1β fragments (F4, F6, F7 and F8) were derived from F0 (panel b). The F4 (349-394) GST-LRP1β fragment was then sub-divided into 7 fragments (F5, F9-F14) (panel b).
(B) Binding of pro-cath-D to GST-LRP1β fragments. Radio-labeled pre-pro-cath-D was incubated with beads containing GST-LRP1β fragments or GST. GST proteins bound to beads were stained by Coomassie (left panels a–c), and bound pre-pro-cath-D was detected by autoradiography (right panels a–c). The binding of pre-pro-cath-D to the fragments derived from F0 is shown in panel a. The binding of pre-pro-cath-D to fragments derived from F4 is shown in panel b. The binding of pre-pro-cath-D after GST-F0 and GST-F4 refolding is shown in panel c. The input (1/10) corresponds to the lysate used for the binding reaction. K, molecular mass in kilodaltons.
