Figure 6. Cath-D interacts with LRP1β in transfected COS cells and in fibroblasts.
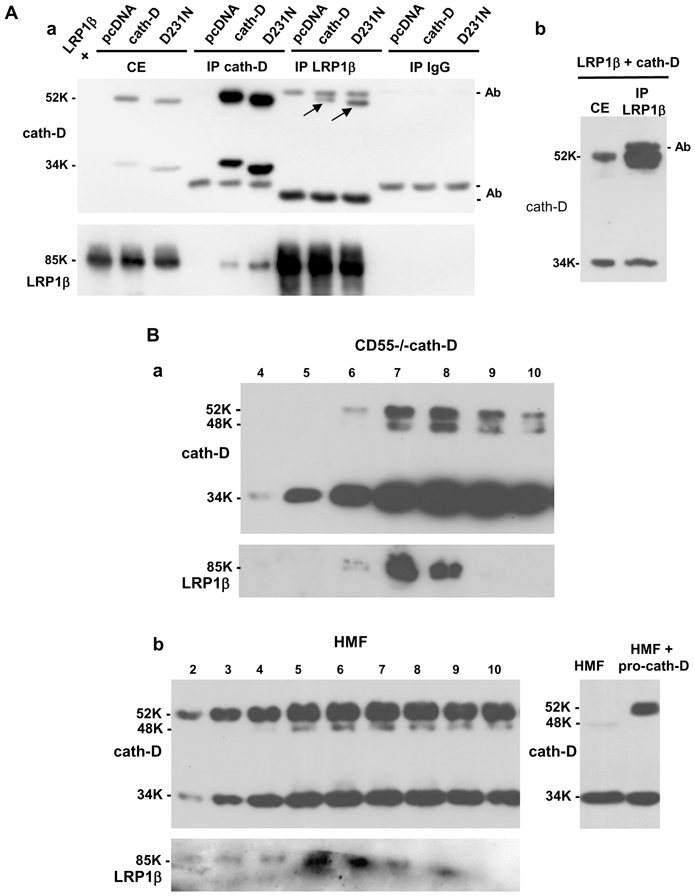
(A) Co-immunoprecipitation of co-transfected pro-cath-D and LRP1β in COS cells. COS cells were transiently co-transfected with LRP1β expression vector, and pcDNA, cath-D, or D231Ncath-D vectors. 48 h post-transfection, unwashed cells were lysed in PLC buffer. Cell extracts (CE), and cath-D, LRP1β and non-immune IgG immunoprecipitations (IP) performed with anti-LRP1β 11H4 hybridoma or anti-cath-D M1G8 antibody were analyzed by anti-cath-D (panel a, top) and anti-LRP1β (panel a, bottom) immunoblotting. Arrows show co-immunoprecipitated pro-cath-D. A longer gel exposure of the LRP1β immunoprecipitation performed in COS cells co-transfected with LRP1β and cath-D vectors and, analyzed by anti-cath-D immunoblotting is shown in panel b.
(B) Co-purification of endogenous LRP1 with cath-D in fibroblasts. Co-purification of endogenous LRP1 with cath-D in cath-D-transfected MEFs (CD55−/−cath-D) (panel a). Cells grown to 90% confluence without medium change for 3 days were directly lysed in PLC buffer, and loaded on an anti-cath-D M1G8 affinity column that binds to 52-, 48-, and 34-kDa forms of cath-D. Eluted fractions were subjected to SDS-PAGE and immunoblotting with the anti-cath-D antibody (top panel) and anti-LRP1β hybridoma (bottom panel). Co-purification of endogenous LRP1 with cath-D in HMF cells treated with pro-cath-D (panel b, left). COS cells were transfected with cath-D, and 48 h post-transfection conditioned medium containing 15 nM of pro cath-D was added to HMF cells. Unwashed HMF fibroblasts incubated for 48 h with conditioned medium containing 15 nM of pro-cath-D were directly lysed in PLC buffer. HMF cell extracts were purified on the M1G8-coupled column and analyzed by immunoblotting as described in panel a. Detection of cath-D in a HMF lysate incubated with or without the conditioned medium containing pro-cath-D is shown in panel b (right). K, molecular mass in kilodaltons.
