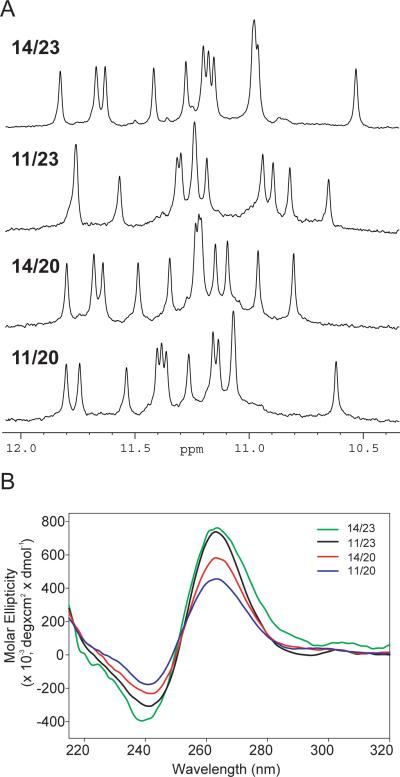
Figure 2.
(A) The imino regions of 1D 1H NMR spectra of the 14/23, 11/23, 14/20, 11/20 sequences which form four loop-isomers of the major c-Myc G-quadruplex. All four modified 22-nt Myc2345 sequences show high quality 1H NMR spectra, indicating the formation of a single and stable G-quadruplex structure. Experimental conditions: 25°C, 30 mM K+-phosphate, 70 mM KCl, pH 7.0, 0.2 mM DNA. (B) CD spectra of 14/23, 11/23, 14/20, 11/20 in the presence of 10 mM Li3PO4 (pH 7) and 2 mM [K+] at 25°C. Each sample contained 10 μM DNA. All four loop-isomers are shown to adopt a parallel-stranded topology with all anti glycosidic sugar conformations for tetrad guanines, as indicated by a positive peak around 265 nm and a negative peak around 240 nm.