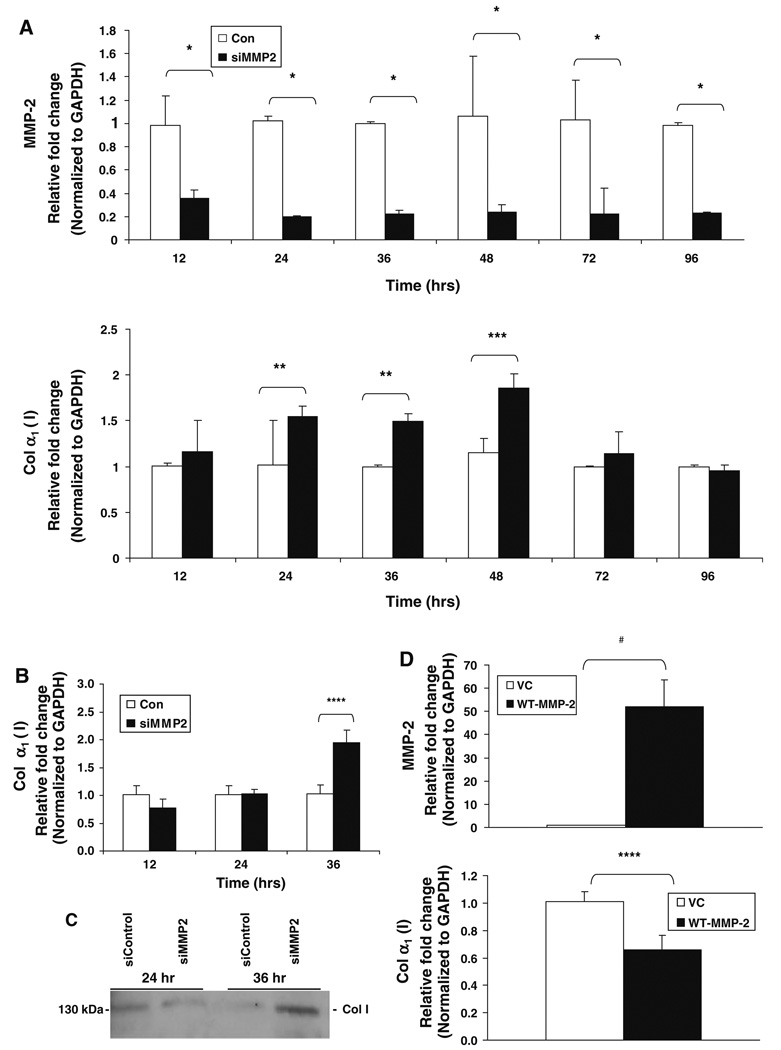
Fig. 6.
Type I collagen expression is regulated by MMP-2 in cell culture. Real-time PCR analysis for MMP-2 and collagen α1(I) mRNA was performed using RNA extracted from LX2 cells, an immortalized human hepatic stellate cell line, after transient transfection with either MMP-2-targeted siRNA (siMMP-2) or nontargeting control siRNA (siControl). MMP-2 is significantly downregulated in LX2 cells at multiple time points after transfection with siMMP-2, as compared with transfection with siControl (a). MMP-2 inhibition with siRNA results in a significant increase in α1(I) collagen mRNA expression at 24, 36, and 48 h (a). Real-time PCR analysis and immunoblot analysis for collagen I expression was performed after transient transfection with siMMP-2 or siControl in passage 3 primary human hepatic stellate cells. A twofold increase in α1(I) collagen mRNA levels was observed 36 h post transfection (b). Culture supernatant was collected and protein was precipitated with acetone. Thirty-five micrograms of protein was loaded per sample. An increased amount of extracellular collagen was observed 36 h after transfection. A representative immunoblot of three independent experiments is shown (c). Real-time PCR analysis for MMP-2 and α1(I) collagen mRNA was performed using RNA extracted from LX2 cells 24 h after transfection with either full-length wild-type MMP-2 (WT-MMP-2) or empty vector control (VC). Overexpression of MMP-2 was associated with a significant decrease in α1(I) collagen mRNA expression (d). All PCR results normalized to GAPDH. Data represent means ± SEM; * P < 0.002, ** P < 0.006, *** P < 0.05, **** P < 0.03, # P < 0.003