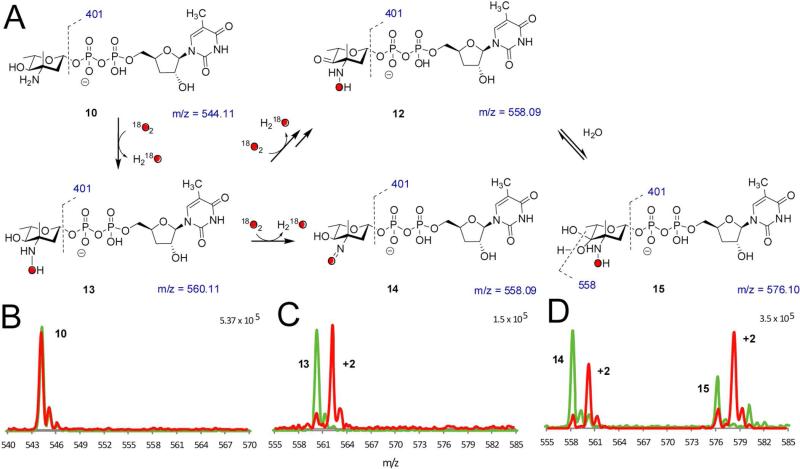
Figure 4.
18O2 incorporation studies. (A) Summary of proposed species observed in 18O2 incorporation experiments. (B – D) 18O2 incorporation with ORF36 and TDP-l-epi-vancosamine 10 incubated with 16O2 (green) and 18O2 (red). Masses shown under structures correspond to unlabeled species. HPLC/MS and MS/MS data were collected at a 15 minutes reaction time, when the hydroxylamine intermediate was most abundant. (B) TDP-l-epi-vancosamine 10 (tR = 4.5 min). (C) hydroxylamine 13 (m/z = 560, tR = 5.3 min). (D) Peaks at tR = 7 – 9 min are proposed to correspond to the nitroso compound 14 (tR = 8.8 min) and an additional oxidation product with m/z = 576 (tR = 7.8 min). This new mass has a distinct retention time from the nitrososugar 14 and, based on MS/MS analysis, the additional mass in the m/z = 576 ion is constrained to the pyranose ring. One possible structure is the 4-keto-3-hydroxylamino sugar 12, which is detected as a hydrate 15 under ESI conditions. MS/MS analysis supports this hypothesis in that the labeled ion m/z = 578 readily fragments to m/z = 560.09 and this fragmentation pattern is identical to the reaction product of 9 shifted by 2 mass units.