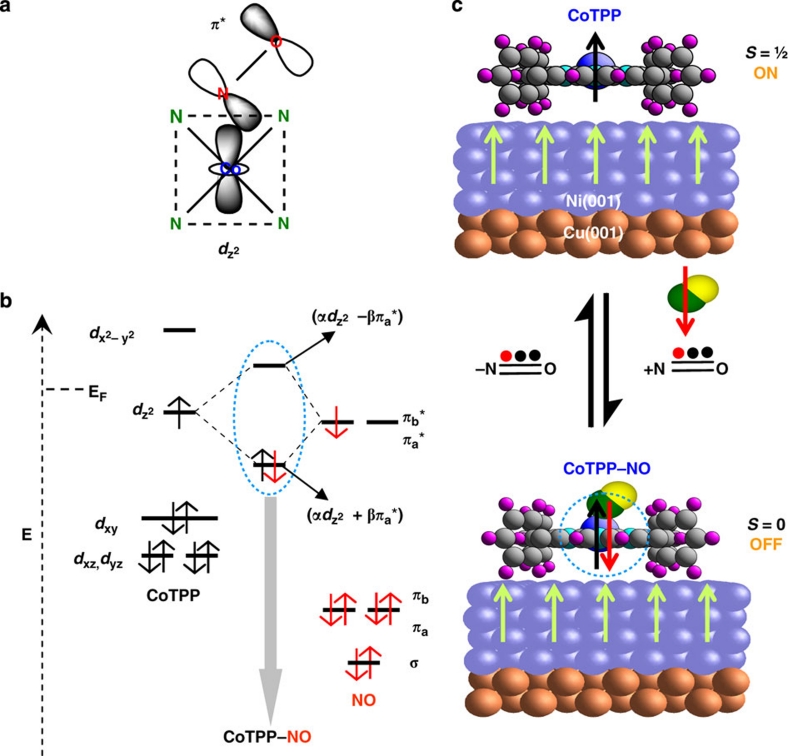
Figure 3. Reversible off–on switching of a molecular spin.
(a) A scheme of the NO–CoTPP complex depicting the involvement of the relevant orbitals. The four green N atoms from the pyrrole moieties of the porphyrin represent the coordination with the Co ion and the dashed lines indicate the Co–4N plane that gets a pyramidal distortion on nitrosyl complex formation. (b) The electronic levels involved in the chemical reaction between NO acting as a π-ligand and CoTPP. Molecular orbital diagrams (the dashed vertical arrow indicates the energy axis, E) of CoTPP (d7 configuration: (dxz)2(dyz)2(dxy)2(dz2)1 (dx2−y2)0 adapted from Wayland et al.34 and Kozuka and Nakamoto35) and NO (configuration: (σ)2(πa)2(πb)2(πa*)1(πb*)0 adapted from Huheey et al.23) showing the presence of unpaired electrons in both CoTPP (dz2) and NO (πa*). On formation of the NO–CoTPP complex (grey arrow), the unpaired spin of CoTPP, which is responsible for the molecular magnetism, is paired up with the unpaired spin supplied by NO in the mixed bonding orbital (highest occupied molecular orbital, HOMO). Consequently, the mixed antibonding orbital (lowest unoccupied molecular orbital, LUMO) is lifted above the Fermi level (EF) of the Ni substrate. Note that the energy levels of the orbitals within the blue dotted circle have been adapted from the literature: Wayland et al.34 and Kozuka and Nakamoto35 for the HOMO/LUMO of the NO–CoTPP complex and Flechtner et al.6 for EF. α and β represent orbital mixing coefficients of dz2 and πa*, respectively. (c) Schematic representation of the switching process: switching on (top)—the CoTPP molecule is ferromagnetically coupled to the Ni substrate and the Co magnetic moment follows the substrate (Ni) magnetization; switching off (bottom)—on addition of NO, CoTPP (S=½) forms the NO–CoTPP complex (S=0) and the spin state of NO–CoTPP remains the same, irrespective of the Ni magnetization. Reversibility is shown by the reaction arrows indicating the chemical reaction with NO and the dissociation of NO.