Abstract
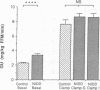
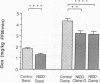
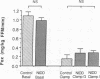
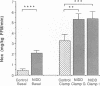
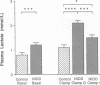
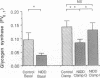
To examine whether reduced rates of oxidative (Gox) and non-oxidative (Nox) glucose metabolism in non-insulin-dependent diabetes mellitus (NIDDM) are due to reduced glucose uptake, intrinsic defects in intracellular glucose metabolism or increased fat oxidation (Fox), indirect calorimetry was performed at similar glucose uptake rates in eight nonobese NIDDM and eight comparable nondiabetic subjects. Three glucose clamp studies were performed: one in the nondiabetic and two in the NIDDM subjects. In the nondiabetic subjects, glucose uptake was increased to 7.62 +/- 0.62 mg/kg of fat-free mass (FFM) per min by increasing serum insulin to 309 pmol/liter at a glucose concentration of 5.1 mmol/liter. By raising the concentration of either serum glucose or insulin fourfold in the NIDDM subjects, glucose uptake was matched to nondiabetic subjects (8.62 +/- 0.49 and 8.59 +/- 0.51 mg/kg FFM per min, respectively, P = NS). Skeletal muscle glycogen synthase activity and plasma lactate levels were measured to characterize Nox. When glucose uptake was matched to nondiabetics by hyperglycemia or hyperinsulinemia, Gox was reduced by 26-28% in NIDDM (P less than 0.025) whereas Fox was similar. Nox was greater in NIDDM (P less than 0.01) and was accompanied by increases in circulating lactate levels. Glycogen synthase activity was reduced by 41% (P less than 0.025) when glucose uptake was matched by hyperglycemia. Glycogen synthase activity was normalized in NIDDM, however, when glucose uptake was matched by hyperinsulinemia. Therefore, a defect in Gox exists in nonobese NIDDM subjects which cannot be overcome by increasing glucose uptake or insulin. Since both glucose uptake and Fox were similar in the two subject groups these factors were not responsible for reduced Gox. Increased Nox in NIDDM is primarily into lactate. Reduced glycogen synthase activity in NIDDM is independent of glucose uptake but can be overcome by increasing the insulin concentration.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG W. D., ENGSTROM A., PAUL K. G. Porphyrins and bone. Scand J Clin Lab Invest. 1962;14 (Suppl 64):7–11. [PubMed] [Google Scholar]
- Arner P., Bolinder J., Engfeldt P., Ostman J. The antilipolytic effect of insulin in human adipose tissue in obesity, diabetes mellitus, hyperinsulinemia, and starvation. Metabolism. 1981 Aug;30(8):753–760. doi: 10.1016/0026-0495(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G., Ray T. K., Smith R. H., Owen O. E. Carbohydrate oxidation and storage in obese non-insulin-dependent diabetic patients. Effects of improving glycemic control. Diabetes. 1983 Nov;32(11):982–987. doi: 10.2337/diab.32.11.982. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C., Thuillez P., Ravussin E., Vasquez B., Narimiga M., Azhar S. Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Invest. 1983 Nov;72(5):1605–1610. doi: 10.1172/JCI111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo B., Napoli R., Di Marino L., Picardi A., Riccardi G., Sacca L. Quantitation of forearm glucose and free fatty acid (FFA) disposal in normal subjects and type II diabetic patients: evidence against an essential role for FFA in the pathogenesis of insulin resistance. J Clin Endocrinol Metab. 1988 Nov;67(5):893–898. doi: 10.1210/jcem-67-5-893. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- Consoli A., Kennedy F., Miles J., Gerich J. Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest. 1987 Nov;80(5):1303–1310. doi: 10.1172/JCI113206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANFORTH W. H. GLYCOGEN SYNTHETASE ACTIVITY IN SKELETAL MUSCLE. INTERCONVERSION OF TWO FORMS AND CONTROL OF GLYCOGEN SYNTHESIS. J Biol Chem. 1965 Feb;240:588–593. [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Ferrannini E., Golay A., Meyer H. U., Theibaud D., Curchod B., Maeder E., Jequier E., DeFronzo R. A. Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes. 1987 Nov;36(11):1341–1350. doi: 10.2337/diab.36.11.1341. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Golay A., Felley C., Jéquier E. Regulation of glucose storage in obesity and diabetes: metabolic aspects. Diabetes Metab Rev. 1988 Nov;4(7):691–700. doi: 10.1002/dmr.5610040706. [DOI] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Finegood D. T., Bergman R. N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987 Aug;36(8):914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- Golay A., DeFronzo R. A., Ferrannini E., Simonson D. C., Thorin D., Acheson K., Thiébaud D., Curchod B., Jéquier E., Felber J. P. Oxidative and non-oxidative glucose metabolism in non-obese type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1988 Aug;31(8):585–591. doi: 10.1007/BF00264764. [DOI] [PubMed] [Google Scholar]
- Golay A., Felber J. P., Jequier E., DeFronzo R. A., Ferrannini E. Metabolic basis of obesity and noninsulin-dependent diabetes mellitus. Diabetes Metab Rev. 1988 Dec;4(8):727–747. doi: 10.1002/dmr.5610040803. [DOI] [PubMed] [Google Scholar]
- Golay A., Felber J. P., Meyer H. U., Curchod B., Maeder E., Jéquier E. Study on lipid metabolism in obesity diabetes. Metabolism. 1984 Feb;33(2):111–116. doi: 10.1016/0026-0495(84)90121-5. [DOI] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Jéquier E., Acheson K., Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- Kochan R. G., Lamb D. R., Reimann E. M., Schlender K. K. Modified assays to detect activation of glycogen synthase following exercise. Am J Physiol. 1981 Feb;240(2):E197–E202. doi: 10.1152/ajpendo.1981.240.2.E197. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LELOIR L. F., OLAVARRIA J. M., GOLDEMBERG S. H., CARMINATTI H. Biosynthesis of glycogen from uridine diphosphate glucose. Arch Biochem Biophys. 1959 Apr;81(2):508–520. doi: 10.1016/0003-9861(59)90232-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Freychet P. Effect of fasting and streptozotocin diabetes on insulin binding and action in the isolated mouse soleus muscle. J Clin Invest. 1979 Nov;64(5):1505–1515. doi: 10.1172/JCI109609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Bogardus C., Mott D. M., Kennedy A. L., Knowler W. C., Howard B. V. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest. 1985 Apr;75(4):1106–1115. doi: 10.1172/JCI111804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Bogardus C. Glucose storage is a major determinant of in vivo "insulin resistance" in subjects with normal glucose tolerance. J Clin Endocrinol Metab. 1986 May;62(5):922–927. doi: 10.1210/jcem-62-5-922. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Madar Z., Kolterman O. G., Bell J. M., Olefsky J. M. Adipocyte glycogen synthase and pyruvate dehydrogenase in obese and type II diabetic subjects. Am J Physiol. 1986 Oct;251(4 Pt 1):E489–E496. doi: 10.1152/ajpendo.1986.251.4.E489. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Meyer H. U., Curchod B., Maeder E., Pahud P., Jequier E., Felber J. P. Modifications of glucose storage and oxidation in nonobese diabetics, measured by continuous indirect calorimetry. Diabetes. 1980 Sep;29(9):752–756. doi: 10.2337/diab.29.9.752. [DOI] [PubMed] [Google Scholar]
- Mott D. M., Lillioja S., Bogardus C. Overnutrition induced decrease in insulin action for glucose storage: in vivo and in vitro in man. Metabolism. 1986 Feb;35(2):160–165. doi: 10.1016/0026-0495(86)90118-6. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Insulin's effect on glucose oxidation independent of glucose transport. Biochem Biophys Res Commun. 1976 Jul 12;71(1):106–113. doi: 10.1016/0006-291x(76)90255-2. [DOI] [PubMed] [Google Scholar]
- Podskalny J. M., Kahn C. R. Insulin activation of glycogen synthase in cultured human fibroblasts is not mediated solely via the insulin receptor. Horm Metab Res. 1986 May;18(5):335–340. doi: 10.1055/s-2007-1012309. [DOI] [PubMed] [Google Scholar]
- Randle P. J. Fuel selection in animals. Biochem Soc Trans. 1986 Oct;14(5):799–806. doi: 10.1042/bst0140799. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Kerbey A. L., Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes Metab Rev. 1988 Nov;4(7):623–638. doi: 10.1002/dmr.5610040702. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Swislocki A. L., Chen Y. D., Golay A., Chang M. O., Reaven G. M. Insulin suppression of plasma-free fatty acid concentration in normal individuals and patients with type 2 (non-insulin-dependent) diabetes. Diabetologia. 1987 Aug;30(8):622–626. doi: 10.1007/BF00277318. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Bogardus C., Kennedy A., Howard B. V. Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest. 1985 Aug;76(2):637–644. doi: 10.1172/JCI112016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Wood T. Distribution of the pentose phosphate pathway in living organisms. Cell Biochem Funct. 1986 Oct;4(4):235–240. doi: 10.1002/cbf.290040402. [DOI] [PubMed] [Google Scholar]
- Wright K. S., Beck-Nielsen H., Kolterman O. G., Mandarino L. J. Decreased activation of skeletal muscle glycogen synthase by mixed-meal ingestion in NIDDM. Diabetes. 1988 Apr;37(4):436–440. doi: 10.2337/diab.37.4.436. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Mott D., Young A. A., Stone K., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. J Clin Invest. 1987 Jul;80(1):95–100. doi: 10.1172/JCI113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. A., Bogardus C., Stone K., Mott D. M. Insulin response of components of whole-body and muscle carbohydrate metabolism in humans. Am J Physiol. 1988 Feb;254(2 Pt 1):E231–E236. doi: 10.1152/ajpendo.1988.254.2.E231. [DOI] [PubMed] [Google Scholar]
- Young A. A., Bogardus C., Wolfe-Lopez D., Mott D. M. Muscle glycogen synthesis and disposition of infused glucose in humans with reduced rates of insulin-mediated carbohydrate storage. Diabetes. 1988 Mar;37(3):303–308. doi: 10.2337/diab.37.3.303. [DOI] [PubMed] [Google Scholar]
- Zawadzki J. K., Wolfe R. R., Mott D. M., Lillioja S., Howard B. V., Bogardus C. Increased rate of Cori cycle in obese subjects with NIDDM and effect of weight reduction. Diabetes. 1988 Feb;37(2):154–159. doi: 10.2337/diab.37.2.154. [DOI] [PubMed] [Google Scholar]