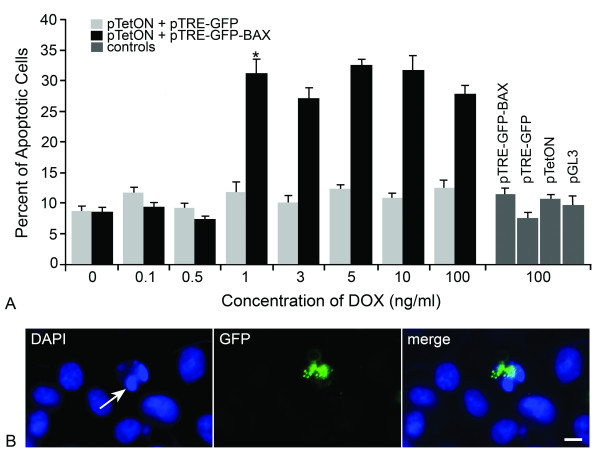
Figure 3.
Indomethacin-induced apoptosis in HCT116Bax-/- cells becomes rapidly saturated with increasing levels of a GFP-BAX fusion protein. HCT116Bax-/- cells were co-transfected with a pTRE-plasmid (carrying either GFP-BAX or GFP) and pTetON, and transgene expression was induced by increasing concentrations of Doxycycline (DOX) in the media. After 24 hrs, cells were treated with indomethacin and cell death assayed 48 hrs later. Cells were also transfected with control plasmids alone and exposed to 100 ng/ml DOX. (A) A histogram showing the percent apoptotic cells 48 hrs after indomethacin treatment at different DOX concentrations (mean ± SEM of 3 separate experiments). Transfection of control vectors alone (pTRE-GFP-BAX, pTRE-GFP, pTetON, and pGL3-Control), or pTetON combined with pTRE-GFP, was observed to elicit 8-10% cell death, indicating that the transfection procedure itself was mildly toxic to these cells. GFP-BAX expression stimulated by 0-0.5 ng/ml DOX was insufficient to cause cell death above these background levels (P = 0.40). At the DOX concentration of 1 ng/ml, however, significant cell death was observed (*P = 6.1 × 10-6, GFP-BAX vs. GFP). Concentrations of DOX greater than 1 ng/ml did not stimulate apoptosis above this level (P = 0.47). (B) A representative photomicrograph of cells transfected with GFP-BAX. DAPI-staining (left panel) showed several cells with normal nuclei and one cell with a fragmented nucleus and condensed chromatin (arrow). GFP imaging of the same field of cells (center) showed one cell expressing the GFP-BAX transgene. GFP-BAX was distributed in a punctate labeling pattern and was localized to the single apoptotic cell in the field (merged image). At this time point after indomethacin treatment, approximately 80% of apoptotic cells were found to be expressing GFP-BAX. Scale bar = 5 μm.