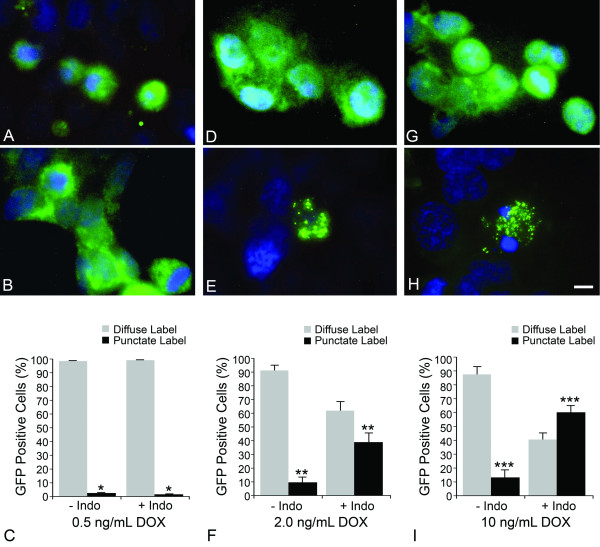
Figure 4.
BAX aggregation is disabled at sub-threshold levels of expression. All photomicrographs of GFP-BAX staining (with DAPI counterstain) were taken 18 hours after vehicle treatment (A, D, G) or indomethacin treatment (B, E, H). Exposure times were adjusted automatically to maximize detection of GFP-BAX localization. As a consequence, the level of fluorescent intensity of the fusion protein in each condition appears similar, even though protein levels linearly increase with increasing doxycycline (DOX) concentration (see Figure 2). (A-C) HCT116 cells expressing a sub-lethal level of GFP-BAX induced by 0.5 ng/ml DOX. (A) GFP-BAX localization was diffuse in cells not exposed to indomethacin. This pattern of distribution remained unchanged in cells after indomethacin treatment (B). (C) Quantitative analysis of GFP-BAX distribution between conditions. There was no significant increase in punctate labeling of GFP-BAX after indomethacin treatment (*P = 0.195). (D-F) HCT116 cells expressing a supra-lethal level of GFP-BAX induced by 2 ng/ml DOX. Addition of indomethacin caused the redistribution of GFP-BAX from a diffuse pattern (D) to a punctate pattern (E). Quantitative analysis (F) of the distribution of GFP-BAX showed a significant shift in the proportion of cells with punctate labeling (**P = 0.0002). (G-I) HCT116 cells expressing a supra-lethal level of GFP-BAX induced by 10 ng/ml DOX. Similar to cells in (D-F), GFP-BAX showed a significant increase in punctate labeling after indomethacin treatment (***P = 8.9 × 10-8). Data for GFP-BAX distribution was collected from a minimum of 100 GFP positive cells per condition and the distribution was scored by 2 masked observers. Scale bar = 5 μm.