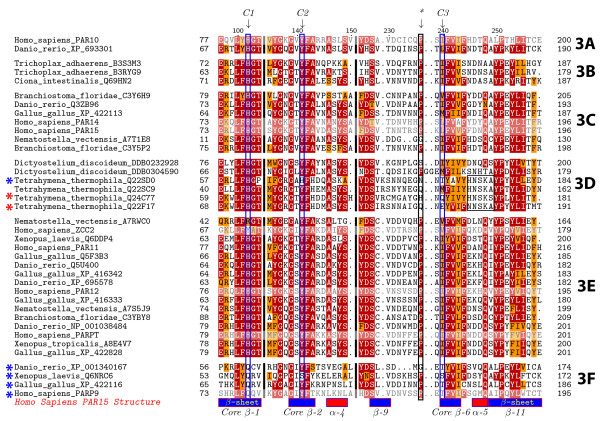
Figure 7.
Clade 3 PARPs have divergent catalytic domains. Alignment of the deduced amino acid sequences of part of the PARP catalytic domains from Clade 3 proteins. Species names and protein accession numbers are shown at left. Numbers indicate amino acid position within each PARP catalytic domain while the labels on the right indicate the subclade to which the sequences belong. Dots indicate gaps introduced to maximize the alignment; the black thick lines indicate missing amino acids introduced to allow representation of all three residues of the catalytic triad, indicated by the blue boxes (C1 = H; C2 = Y; C3 = E). Only one Clade 3 protein contains a glutamic acid residue at the third position, while another has a glutamine (both indicated with red asterisks). Most Clade 3 proteins have substituted aliphatic amino acids (no asterisk), while five have serine or threonine at the position of the glutamic acid (blue asterisks). The black box surrounds a short motif characteristic of Tetrahymena thermophila Clade 3 proteins. The black box labelled with an asterisk indicates a proline residue that is found in most of the Clade 3 proteins. Shaded sequences indicate proteins for which a 3D structure is available.